Org. Synth. 2010, 87, 288
DOI: 10.15227/orgsyn.087.0288
SAFE AND SCALABLE PREPARATION OF BARLUENGA'S REAGENT
[Bis(pyridine)iodonium(I) tetrafluoroborate]
Submitted by Justin M. Chalker, Amber L. Thompson, and Benjamin G. Davis
1.
Checked by Nilesh Zaware and Peter Wipf
2.
1. Procedure
A. Silver tetrafluoroborate on SiO2. A 500-mL, one-necked round-bottomed flask is equipped with a Teflon-coated magnetic stir bar (3.9 cm × 1 cm, rectangular). Deionized water (50
mL) is poured into the flask and tetrafluoroboric acid (6.3 mL, 50 mmol, 1.0 equiv) is added by syringe (Note 1). The solution is stirred, open to air, at room temperature (23 °C) for 2 min and silver carbonate (6.89 g, 25.0 mmol, 0.50 equiv) is added in several portions with a spatula over 2 min (Note 2). Carbon dioxide gas evolves. The resulting mixture is stirred rapidly (380 rpm) until all solids are dissolved to give a grey solution (20 min). Silica gel (10.0 g) is poured into the solution and the mixture is stirred for 5 min (Note 3). The stir bar is removed using a magnetic wand, rinsed with deionized water (1
mL) with a glass pipette, and the flask is transferred to a rotary evaporator into a water bath pre-heated to 55 °C. The water in the reaction flask is evaporated (55 °C, 80 mmHg, cooling is effected by circulating chilled water through the condenser) over 35 min to give the AgBF4 on SiO2 as an off-white/grey solid on the walls of the flask. A spatula is used to scrape all solids to the bottom of the flask, which is then returned to the rotary evaporator and dried with rapid spinning (55 °C, 80 mmHg). After 15 min of drying in this manner, a spatula is used to scrape all solids to the bottom of the flask. A free flowing powder is obtained. The flask is allowed to stand and cool at room temperature for 5 min before the mixture is used directly in the next step.
B. Bis(pyridine)iodonium(I) tetrafluoroborate (IPy2BF4, 1). A Teflon-coated magnetic stir bar (3.9 cm × 1 cm, rectangular) is added to the 500-mL 1-necked flask that contains the AgBF4 on SiO2 from above. Dichloromethane (300
mL) is poured into the flask and the mixture is stirred at room temperature, open to the atmosphere (Note 4). Pyridine (8.1 mL, 100 mmol, 2.0 equiv) is poured into the reaction flask from a 10.0 mL measuring cylinder, and the mixture is stirred at room temperature (Note 5). One minute after the addition of pyridine, iodine flakes (12.69 g, 50.0 mmol, 1.0 equiv) are poured into the reaction flask from a weighing paper (Note 6). A yellow precipitate (AgI) is formed immediately upon addition of the iodine. A glass stopper is used to loosely cap the reaction flask and the mixture is stirred vigorously (380 rpm) for 1 h (Note 7). The solution gradually turns red as the iodine is dissolved. After 1 h of stirring, the solids are removed by filtration through a sintered glass funnel (Note 8) under suction (15 mmHg), and the dark red filtrate is collected directly in a 1-L, 1-necked round-bottomed flask using a 24/40 glass filtration adapter. The reaction flask is rinsed with dichloromethane (100
mL) to ensure complete transfer, and the SiO2/AgI filter cake is then washed with additional dichloromethane (100
mL). The solvent is removed by rotary evaporation (35 °C, 80 mmHg) to give a reddish-yellow solid. This solid is then dissolved in dichloromethane (100
mL) and a Teflon-coated stir bar (5 cm × 1 cm, rectangular) is added. The solution is stirred in an ice bath for 5 min and then diethyl ether (200
mL) is poured into the stirred solution to precipitate the product (Note 9). The mixture is stirred at 0 °C for 10 min to ensure that all IPy2BF4 is precipitated. The product is isolated by filtration under suction (15 mmHg) (Note 10). The stirrer is removed by a magnetic wand, held over the filter funnel and washed with diethyl ether (4) using a glass pipette. The flask is rinsed with diethyl ether (50
mL) to complete the transfer. The off-white powder is dried on the filter with suction for 5 min, transferred to a weighing paper (6" × 6") and dried under vacuum in a desiccator for 4 h (1.2 mmHg) to obtain 1 (13.67 g, 73%) (Notes 11, 12, 13 and 14). This material is used for NMR, IR and Mp analysis. IPy2BF4 can also be recrystallized from dichloromethane. Accordingly, the powdered 1 (13.64 g) is added to a 100-mL, 1-necked round-bottomed flask with a Teflon-coated stir bar (1.5 cm × 1 cm, rectangular). A water bath is preheated to 50 °C and dichloromethane (25
mL) is added to the solid. The suspension is stirred (870 rpm) and lowered into the water bath. Once the dichloromethane is boiling (ca. 1 min), additional dichloromethane is added by glass pipette, with stirring, until all solids are dissolved (48 mL of additional dichloromethane, ca. 73 mL of total solvent). Once a clear solution is obtained, the stirring is stopped and the flask removed from the water bath. Crystals are observed within 3 min. After 30 min, the flask is cooled to 0 °C (ice bath) to facilitate the crystallization. After 2 h on ice, the crystals are isolated by filtration with suction (15 mmHg) (Note 15), collecting the filtrate directly in a 250-mL round-bottomed flask. The suction is removed, the stir bar is held over the filter funnel with a magnetic wand and washed with diethyl ether (2
mL), and the crystals are triturated with diethyl ether (30
mL) on the filter. The crystals are then dried on the filter for 5 min with suction, transferred to a jar and dried under vacuum in a desiccator for 2 h (1.2 mmHg). The first crop of crystals yields 8.34 g (Notes 16 and 17). All remaining material in the crystallization flask and filter is collected by rinsing with dichloromethane (50
mL). The combined filtrates and mother liquor are concentrated by rotary evaporation (35 °C, 80 mm Hg) to give a reddish yellow solid. The recrystallization is repeated twice more to recover the remaining material, using dichloromethane (24
mL) in a 50-mL round-bottomed flask in the second recrystallization (2.46 g of crystals isolated) and dichloromethane (23
mL) in a 25-mL round-bottomed flask in the third and final recrystallization (0.79 g of crystals isolated). The total recovery of 1 from recrystallization is 11.59 g (85% from powdered IPy2BF4 and 62% overall).
2. Notes
1.
The submitters utilized deionized water from an Elix 10 water purification system equipped with a Progard 1 pretreatment pack (Millipore). The checkers used deionized water from a Barnstead purification system. Tetrafluoroboric acid (8.0 M, 49.5-50.5% w/w) was purchased from Fluka and used as received. The checkers bought fluoroboric acid (50 wt% solution in water) from Acros Organics and used it as received.
2.
Silver carbonate (99%) was purchased from Alfa Aesar and used as received. The checkers bought silver carbonate (99.5%) from Alfa Aesar and used it as received.
3.
The submitters purchased SiO
2 (10% <40 μm and 10% >63 μm, Laboratory Reagent Grade for Flash Chromatography) from VWR and used it as received. Checkers purchased SiO
2 (40-63 μm (230-400 mesh) Siliaflash
® P60) from Silicycle and used it as received.
4.
Dichloromethane (HPLC grade) was purchased from Fisher Scientific and used as received.
5.
Submitters used pyridine (glass distilled grade) purchased from Rathburn Chemicals Ltd. as received. Checkers used pyridine (GR grade) purchased from EMD Chemicals as received.
6.
Iodine beads (laboratory reagent grade) were purchased from Fisher Scientific and used as received. The checkers bought iodine (flakes, certified ACS) from Fisher Scientific.
7.
The formation of the product, IPy
2BF
4, is difficult to monitor by conventional methods such as TLC. Qualitatively, the formation of AgI (yellow solid) and the dissolution of the iodine beads are indicators of reaction progression. Typically, 1 h of stirring is sufficient for a preparation on the scale described, and prolonged reaction time did not improve the final yield.
8.
The submitters used a Büchner filter funnel (porosity 3 glass frit) with a 250 mL capacity for the filtration. The checkers used a Büchner filter funnel (coarse, porosity 40-60 glass frit) with a 150 mL capacity for the filtration.
9.
The submitters used diethyl ether (glass distilled grade) purchased from Rathburn Chemicals Ltd. as received. The checkers used diethyl ether (GR grade) purchased from EMD Chemicals as received.
10.
The submitters used a Büchner filter funnel (porosity 3 glass frit) with a 250 mL capacity layered with filter paper (QL 100 from Fisher Scientific) for the isolation of IPy
2BF
4. The checkers used a Büchner filter funnel (porosity 10-15 glass frit) with a 150 mL capacity for the isolation of IPy
2BF
4. The checkers did not use a filter paper.
11.
The preparation was run in triplicate on the described scale by the submitters with yields of 13.0 to 13.6 g (70-73%) after precipitation with diethyl ether.
12.
As observed by the submitters, the powdered IPy
2BF
4 is sufficiently pure for most synthetic applications and can be stored for months if protected from light and kept at -20 °C.
13.
Working at 50% scale, the checkers obtained 5.65 g (61%).
14.
Spectroscopic data obtained for
1 prior to recrystallization: Mp: 151-173 °C (Lit
3: 149-151 °C); IR (ATR) 1600, 1453, 1062, 786, 659 cm
-1;
1H NMR
pdf (300 MHz, CD
3CN) δ: 7.64 (dd,
J = 7.8, 6.6 Hz, 4 H), 8.26 (tt,
J = 7.8, 1.5 Hz, 2 H), 8.79 (dd,
J = 6.3, 1.2 Hz, 4 H);
13C NMR
pdf (75 MHz, CD
3CN) δ: 127.9, 142.3, 149.7;
19F NMR
pdf (282 MHz, CD
3CN) δ: -151.65, -151.60.
15.
The submitters used a Büchner filter funnel (porosity 3 glass frit) with a 250 mL capacity, layered with a filter paper (QL 100 from Fisher scientific) for the isolation of IPy
2BF
4. The checkers used a Büchner filter funnel (porosity 4-5.5 glass frit) with a 60 mL capacity. Checkers did not use a filter paper.
16.
Analytical data obtained for recrystallized
1: Mp: 151-179 °C (Lit
3: 149-151 °C);
19F NMR (282 MHz, CD
3CN) δ: -151.81, -151.76; Anal. calcd. for C
10H
10BF
4IN
2: C, 32.29; H, 2.71; N, 7.53; found: C, 32.26; H, 2.52; N, 7.56;
1H NMR data matched data listed in
Note 14.
17.
Submitters prepared single crystals (from the first crop of crystals) suitable for X-ray analysis to unambiguously confirm the identity of the product. Accordingly, 500 mg of the first crop of crystals was dissolved in 3.0 mL of boiling dichloromethane in a straight-sided glass tube (2.0 × 5.0 cm) and left to cool, open to air. After 30 min, the vial was capped, covered in foil, and left to stand overnight at room temperature (23 °C). Colorless plate-like crystals were isolated from the mother liquor and coated in inert perfluoro-polyether oil, and mounted on a hair and frozen in place at 150 K using an Oxford Cryosystems Cryostream N2 open flow cooling device.
4 Diffraction data were collected using a Nonius Kappa-CCD diffractometer and results compared with those reported by Alvarez-Rua et al.
5 Three crystals were tested in all by the collection of a 10° ω-scan which indexed in each case to give the same monoclinic cell (by transformation, similar to that reported previously
6; see supplementary information, for details). A complete dataset to a maximum resolution of 0.77 Å was recorded for the third crystal. On completion, the crystal was warmed to 250 K at 120 K/h, where the cell was redetermined and found to be a smaller, related C-centered monoclinic cell. A second dataset was recorded for comparison before the crystal was returned to 150 K (at 120 K/h) where the cell was found to have returned to that seen previously at that temperature, demonstrating the occurrence of a reversible structural phase transition. Cell parameters and intensity data for both data sets were processed using the DENZO-SMN package
7 and the structures solved by direct methods using SIR92.
8 Both structures were refined by full-matrix least squares on F
2 using the CRYSTALS suite (Figure 1).
9 Where possible, non-hydrogen atoms were refined with anisotropic displacement parameters and the hydrogen atoms were initially refined with soft restraints, then constrained using a riding model. In the case of the 250 K data, the BF
4 counter-ion was found to be disordered and a full description of the way this was modeled and a brief comparison of the structures is given in the supplementary information, together with the structural data in CIF format. These data have also been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC 772585 and CCDC 772586, respectively, and copies can be obtained, free of charge from The Cambridge Crystallographic Data Centre via www. ccdc.cam.ac.uk/data_request/cif.
Figure 1. The molecular structure of bis(pyridine)iodonium(I) tetrafluoroborate, from the crystal structure at 150 K. Only one molecule is shown for clarity and the atomic displacement parameters are drawn at 50% probability.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Bis(pyridine)iodonium(I) tetrafluoroborate (IPy
2BF
4,
1) - also referred to as Barluenga's Reagent, in recognition of its discoverer
3 - is a mild iodonium source and oxidant. IPy
2BF
4 is a relatively stable solid and is soluble in both organic and aqueous systems. The utility of
1 in organic synthesis has been widely demonstrated and a selection of transformations promoted by IPy
2BF
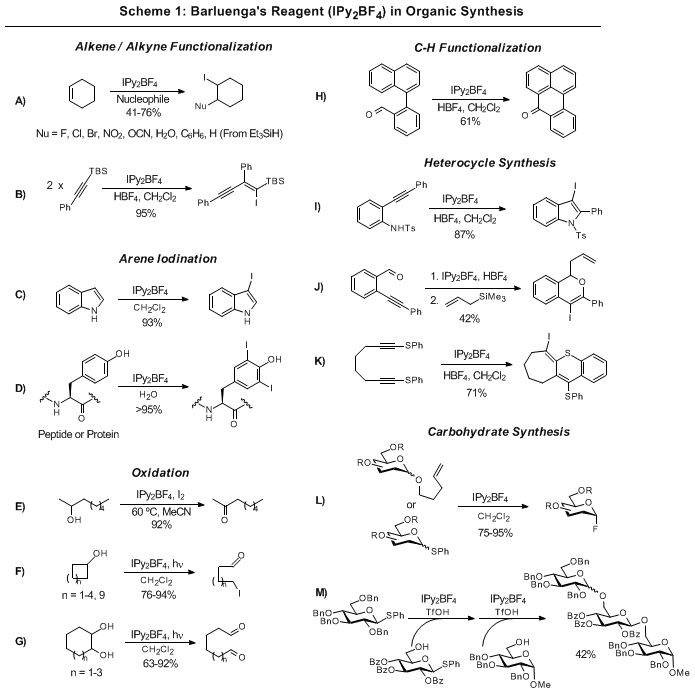
4 is shown in Scheme 1. Alkenes, alkynes, and arenes are iodinated by
1 under mild conditions (Scheme 1A-D).
3,10,11 This method is mild enough that selective iodination of tyrosine in peptides
12 and proteins
13 has been realized. Barluenga's reagent can oxidize secondary alcohols to ketones under thermal conditions (Scheme 1E). Under photolytic conditions, cycloalkanols are oxidatively cleaved to the corresponding ω-iodocarbonyls when treated with
1, and 1,2-diols are converted to the respective dicarbonyls (Scheme 1F-G).
14,15 C-H functionalization
16 and heterocycle synthesis
17,18,19 is also enabled by
1 (Scheme 1H-K). Additionally, IPy
2BF
4 is useful in carbohydrate synthesis (Scheme 1L-M). Thio- and
n-pentenyl glycosides can be activated for glycosylation or converted to the corresponding glycosyl fluoride with
1.
20,21 Finally, it is noteworthy that many of the reactions in Scheme 1 involve carbon-carbon bond formations (A, B, H, J, K).
Given the synthetic versatility of IPy
2BF
4, it is useful to have ready access to multi-gram quantities of this reagent. Even though Barluenga's reagent is available from several commercial suppliers, the cost may preclude its use in large-scale synthetic endeavors.
22 Moreover, the preparation of
1described in the literature uses extremely toxic Hg(II) salts that necessitate a risky and tedious workup.
3,23 The preparation above is adapted procedurally from Barluenga's initial reports, but replaces Hg(II) with Ag(I).
24 The resulting procedure is a much safer and convenient synthesis, with yields comparable to that reported by Barluenga. The protocol reported herein can conveniently supply more than 10 g of IPy
2BF
4 in less than a day. With a safe, scalable synthesis of IPy
2BF
4 in hand, we anticipate increased deployment of Barluenga's reagent in synthetic endeavors.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Tetrafluoroboric acid: Borate(1-), tetrafluoro-, hydrogen (1:1); (16872-11-0)
Silver carbonate: Carbonic acid, silver(1+) salt (1:2); (534-16-7)
Pyridine: (110-86-1)
Iodine: (7553-56-2)
Bis(pyridine)iodonium(I) tetrafluoroborate: Iodine(1+), bis(pyridine)-, tetrafluoroborate(1-) (1:1); (1 5656-28-7)
 |
Benjamin G. Davis completed his B.A. (1993) and D.Phil. (1996) at the University of Oxford. During this time he learned the beauty of carbohydrate chemistry under the supervision of Professor George Fleet. He is now a full Professor at the University of Oxford and Pembroke College. His group's research centers on chemical biology with an emphasis on carbohydrates and therapeutic proteins. In 2008, he was awarded the Wain Medal for Chemical Biology and was the first U.K. recipient of the American Chemical Society's Horace S. Isbell Award. Ben is also a 2009-2010 Novartis Chemistry Lecture Award recipient. |
 |
Justin M. Chalker was born in Kansas City, Kansas in 1983. He graduated from the University of Pittsburgh with a B.S. in Chemistry and a B.A. in the History and Philosophy of Science in 2006. Justin carried out undergraduate research in the laboratory of Prof. Theodore Cohen where he investigated the Zn-ene cyclization and its use in total synthesis. As a Rhodes Scholar, Justin is pursuing his Ph.D. at the University of Oxford under the direction of Prof. Benjamin G. Davis where he is exploring novel aqueous chemistry for selective protein modification. |
 |
Amber L. Thompson graduated from University of Durham with B.Sc. in chemistry in 2000. She went on to complete her Ph.D. on "Structure-Property Correlations in Novel Spin Crossover Materials" under the supervision of Dr. Andrés Goeta, also at Durham. After a short time working with Prof. Judith Howard, she moved to Oxford, where she runs the Chemical Crystallography Service, teaching chemists to solve crystal structures and working on the wide range of problems they encounter. |
 |
Nilesh Zaware obtained his B. Pharm. (2002) from the University of Mumbai, India. He then moved to Duquesne University to pursue his Ph.D. in Medicinal Chemistry under the supervision of Professor Aleem Gangjee, where his research was focused on the synthesis of pyrrolo[2,3-d]pyrimidines and pyrimido[4,5-b]indoles as inhibitors of multiple receptor tyrosine kinases, folate metabolizing enzymes and tubulin. After completion of his Ph.D. in 2009 he joined the group of Professor Peter Wipf at the Department of Chemistry and Center for Chemical Methodologies & Library Development at the University of Pittsburgh as a postdoctoral research associate and is currently working on the synthesis of structurally and stereochemically diverse tetrahydropyrans. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved