Org. Synth. 1922, 2, 79
DOI: 10.15227/orgsyn.002.0079
QUINOLINE
Submitted by H. T. Clarke and Anne W. Davis.
Checked by Roger Adams and A. W. Sloan.
1. Procedure
In a 5-l. round-bottomed flask, fitted with an efficient reflux condenser of wide bore, are placed, in the following order, 80 g. of powdered crystalline ferrous sulfate (Note 1), 865 g. (687 cc., 9.4 moles) of c.p. glycerol (Note 2), 218 g. (213 cc., 2.3 moles) of aniline, 170 g. (141 cc., 1.4 moles) of nitrobenzene, and 400 cc. of concentrated sulfuric acid (sp. gr. 1.84) (Note 3). The contents of the flask are well mixed and the mixture heated gently over a free flame. As soon as the liquid begins to boil, the flame is removed, since the heat evolved by the reaction is sufficient to keep the mixture boiling for one-half to one hour. If the reaction proceeds too violently at the beginning, the reflux condenser may be assisted by placing a wet towel over the upper part of the flask. When the boiling has ceased the heat is again applied and the mixture boiled for five hours. It is then allowed to cool to about 100° and transferred to a 12-l. flask; the 5-l. flask is rinsed out with a small quantity of water.
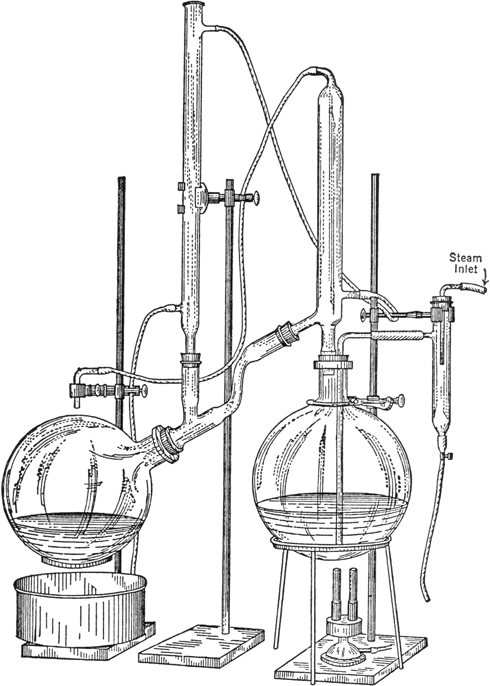
The 12-l. flask is then connected with the steam-distillation apparatus shown in Fig. 24, a 12-l. flask being used as a receiver (Note 4); steam is passed in (without external heat) until 1500 cc. has distilled (ten to thirty minutes). This removes all the unchanged nitrobenzene (10–20 cc.). The current of steam is then interrupted, the receiver is changed, and 1.5 kg. of 40 per cent sodium hydroxide solution is added cautiously through the steam inlet. The heat of neutralization is sufficient to cause the liquids to boil and thus become thoroughly mixed. Steam is then passed in as rapidly as possible until all the quinoline has distilled. In this process, 6–8 l. of distillate is collected (two and one-half to three and one-half hours are required, unless a very efficient condensing apparatus is used, under which conditions the distillation may be complete in one-half to one and one-half hours). The distillate is allowed to cool, and the crude quinoline separated. The aqueous layer of the distillate is again distilled with steam until all the quinoline has been volatilized and collected in about 3 l. of distillate.
Fig. 24.
This 3 l. of distillate is then mixed with the first yield of quinoline, and 280 g. (150 cc.) of concentrated sulfuric acid is added. The solution is cooled to 0–5°, and a saturated solution of sodium nitrite added until a distinct excess of nitrous acid is present (as shown either by starch-potassium iodide paper or by the odor). This generally requires 50 to 70 g. of sodium nitrite. The mixture is then warmed on a steam bath for one hour, or until active evolution of gas ceases, and is then distilled with steam until all the volatile material has been expelled (4 l. of distillate will result). The receiver is then changed and the mixture in the distillation flask is neutralized, as before, with 700 g. of 40 per cent sodium hydroxide solution. The quinoline is distilled exactly as described above, the aqueous portions of the distillate being distilled with steam until all the quinoline has been isolated. The crude product is then distilled under reduced pressure, and the fraction which boils at 110–114°/14 mm. is collected. The forerun is separated from any water which may be present, dried with a little solid alkali, and redistilled. The total yield is 255–275 g. (84–91 per cent of the theoretical amount based on the aniline taken) (Note 5).
2. Notes
1.
In the Skraup synthesis of
quinoline the principal difficulty has always been the violence with which the reaction generally takes place; it occasionally proceeds relatively smoothly, but in the majority of cases gets beyond control, with consequent loss of material through the condenser. By the addition of
ferrous sulfate, which appears to function as an
oxygen carrier, the reaction is extended over a longer period of time. It is thus possible to work with much larger quantities of material when
ferrous sulfate is employed.
2.
In a number of experiments, the
glycerol used contained an appreciable amount of water. Under these conditions, the yield of product is much lower. "Dynamite"
glycerol containing less than one-half per cent of water is best employed; u.s.p.
glycerol contains 5 per cent of water and usually gives lower yields.
3.
It is important that the materials should be added in the correct order; should the
sulfuric acid be added before the
ferrous sulfate, the reaction may start at once. It is also important to mix the materials well before applying heat; the
aniline sulfate should have dissolved almost completely, and the
ferrous sulfate should be distributed throughout the solution. To avoid danger of overheating, it is well to apply the flame away from the center of the flask where any solids would be liable to congregate.
4.
The apparatus for steam distillation shown in Fig. 24 requires little desk space. In this apparatus the greater portion of the condensation is effected by the stream of water passing over the receiver. It is, therefore, necessary that the stream passing through the condenser should be sufficiently rapid to cause it to form a uniform film over the receiving flask.
Much time can be saved by the use of the steam distillation apparatus described, especially when large quantities have to be handled. The above directions avoid the use of extraction methods, which not only consume more time but may lead to appreciable losses of material.
5.
Although these directions have been used many times with results exactly as described, in a few cases the yields have dropped to
60–65 per cent without any apparent reason. At present no explanation can be given for this.
The percentage yields have been based on the amount of aniline taken. It would probably be more legitimate to base the calculation on the amounts of aniline taken and of nitrobenzene not recovered, since undoubtedly the latter is reduced to aniline during the course of the reaction. If this is done, the yield is found to be only 55 to 60 per cent of the calculated amount.
3. Discussion
Quinoline can be prepared by heating a mixture of
aniline,
glycerol, and
sulfuric acid1 alone or with an oxidizing agent like
nitrobenzene,
2 arsenic acid,
3 ferric oxide,
4 and
vanadic acid.
5 With the use of
nitrobenzene, the reaction, according to the original method, takes place with extreme violence. The procedure followed here gives higher yields than those obtained with the
ferric oxide method
4 and is the most satisfactory for the preparation of
quinoline, but its homologs are preferably prepared by the use of
arsenic acid because of the somewhat greater yields. The violence of the original
nitrobenzene method may also be moderated by the use of
acetic6 or boric7 acid.
Copper sulfate has been used as a catalyst in the Skraup synthesis,
8 and the
iron salt of m-nitrobenzenesulfonic acid has been employed as the oxidizing agent.
9 Preliminary experiments on the
boric acid method showed that the reaction runs smoothly but gives yields somewhat lower than those reported.
7
This preparation is referenced from:
- Org. Syn. Coll. Vol. 1, 123
- Org. Syn. Coll. Vol. 1, 162
- Org. Syn. Coll. Vol. 1, 170
- Org. Syn. Coll. Vol. 1, 175
- Org. Syn. Coll. Vol. 1, 304
- Org. Syn. Coll. Vol. 1, 318
- Org. Syn. Coll. Vol. 1, 511
- Org. Syn. Coll. Vol. 1, 514
- Org. Syn. Coll. Vol. 2, 217
- Org. Syn. Coll. Vol. 2, 474
- Org. Syn. Coll. Vol. 2, 569
- Org. Syn. Coll. Vol. 3, 6
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
alkali
sulfuric acid (7664-93-9)
aniline (62-53-3)
sodium hydroxide (1310-73-2)
glycerol (56-81-5)
oxygen (7782-44-7)
copper sulfate (7758-98-7)
sodium nitrite (7632-00-0)
nitrous acid (7782-77-6)
ferrous sulfate (13463-43-9)
arsenic acid (1327-52-2)
Nitrobenzene (98-95-3)
Quinoline (91-22-5)
boric acid (10043-35-3)
aniline sulfate
ferric oxide (1309-37-1)
vanadic acid
iron salt of m-nitrobenzenesulfonic acid
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved