Org. Synth. 1969, 49, 81
DOI: 10.15227/orgsyn.049.0081
METHYL KETONES FROM CARBOXYLIC ACIDS: CYCLOHEXYL METHYL KETONE
[Ketone, cyclohexyl methyl]
Submitted by Thomas M. Bare and Herbert O. House
1.
Checked by A. De Meijere and K. B. Wiberg.
1. Procedure
Caution! Since hydrogen is liberated in the first step of this reaction, it should be conducted in a hood. A dry, 500-ml., three-necked flask is fitted with a reflux condenser, a pressure-equalizing dropping funnel, a mechanical stirrer, and an inlet tube to maintain a static nitrogen atmosphere in the reaction vessel throughout the reaction. In the flask are placed 1.39 g. (0.174 mole) of powdered lithium hydride (Note 1) and 100 ml. of anhydrous 1,2-dimethoxyethane (Note 2). While this suspension is stirred vigorously, a solution of 19.25 g. (0.150 mole) of cyclohexanecarboxylic acid (Note 3) in 100 ml. of anhydrous 1,2-dimethoxyethane (Note 2) is added dropwise over a 10-minute period. The resulting mixture is heated to reflux with stirring for 2.5 hours, at which time hydrogen evolution and the formation of lithium cyclohexanecarboxylate are complete. After the resulting suspension has been cooled to approximately 10° with an ice bath, it is stirred vigorously while 123 ml. of an ethereal solution containing 0.170 mole of methyllithium (Note 4) is added dropwise over a 30-minute period. After the addition is complete, the ice bath is removed and the resulting suspension is stirred at room temperature for 2 hours. The dropping funnel is removed from the reaction flask and replaced by a rubber septum fitted with a 4-mm. O.D. glass tube of suitable dimensions to permit the reaction mixture to be siphoned from the reaction flask when a slight positive nitrogen pressure is present in the flask.
The fine suspension in the reaction flask is agitated and siphoned into a vigorously stirred mixture of 27 ml. (0.32 mole) (Note 5) of concentrated hydrochloric acid and 400 ml. of water. The reaction flask is rinsed with an additional 100 ml. of ether which is also added to the aqueous solution. After the resulting mixture has been saturated with sodium chloride, the organic phase is separated and the alkaline (Note 5) aqueous phase is extracted with three 150-ml. portions of ether. When the combined organic solutions have been dried over magnesium sulfate, the bulk of the ether is distilled from the mixture through a 40-cm. Vigreux column (Note 6) and then the residual ether and the 1,2-dimethoxyethane are distilled from the mixture through a 10-cm. Vigreux column. Distillation of the residual pale yellow liquid separates 17.1–17.7 g. (91–94%) of the methyl ketone as a colorless liquid, b.p. 57–60° (8 mm.), n26D 1.4488–1.4489 (Note 7).
2. Notes
1.
Lithium hydride of suitable quality may be purchased from Alfa Inorganics, Inc., 8 Congress Street, Beverly, Massachusetts 01915.
2.
Commercial 1,2-dimethoxyethane, b.p. 85–86°, purchased from Eastman Organic Chemicals, was distilled from lithium aluminum hydride before use.
3.
The
cyclohexanecarboxylic acid, m.p. 31–32°, purchased from Aldrich Chemical Company was used without further purification.
4.
An ethereal solution which was 1.38
M in
methyllithium was purchased from Foote Mineral Company. The concentration of
methyllithium in ethereal solutions may be conveniently determined by a procedure described elsewhere
2,3 in which the
lithium reagent is titrated with
sec-butyl alcohol, utilizing the charge transfer complex formed from
bipyridyl or
o-
phenanthroline and the
lithium reagent as an indicator.
5.
The quantity of
hydrochloric acid used is normally insufficient to neutralize all the
lithium hydroxide produced when the reaction mixture is quenched in the aqueous solution. As a result, any unchanged
cyclohexanecarboxylic acid will be present as its lithium salt and will remain in the aqueous phase.
6.
The product is sufficiently volatile that use of a
rotary evaporator or an
open flask to distill the bulk of the
ether and
1,2-dimethoxyethane from this solution may result in loss of a significant fraction of the product.
7.
The product may be analyzed by use of a
gas chromatography column packed with either LAC-728 (diethylene glycol succinate) or Carbowax 20M suspended on Chromosorb P. Using a 2.5-m. LAC-728 column heated to 100°, the submitters found retention times of 9.4 and 13.0 minutes for
cyclohexyl methyl ketone and
cyclohexyldimethylcarbinol. Less than 1% of the carbinol by-product was present.
3. Discussion
Apart from the reaction of
cyclohexanecarboxylic acid with
methyllithium,
4 cyclohexyl methyl ketone has been prepared by the reaction of cyclohexylmagnesium halides with
acetyl chloride or
acetic anhydride5,6,7 and by the reaction of
methylmagnesium iodide with
cyclohexanecarboxylic acid chloride.
8 Other preparative methods include the
aluminum chloride-catalyzed acetylation of
cyclohexene in the presence of
cyclohexane,
9 the oxidation of
cyclohexylmethylcarbinol,
10,11 the decarboxylation and rearrangement of the glycidic ester derived from
cyclohexanone and
t-butyl α-chloropropionate,
12 and the catalytic hydrogenation of
1-acetylcyclohexene.
13,14This preparation illustrates the procedure for reaction of organolithium reagents with the lithium salts of carboxylic acids to form ketones.
15 The reaction is generally applicable to alkyl, vinyl, and aryl organolithium reagents and carboxylic acid salts which do not contain other interfering functional groups. However, the reaction has been most often used with
methyllithium for the preparation of methyl ketones. The reaction is known to effect the
stereospecific conversion of a carboxylic acid to methyl ketone and, consequently, is a useful part of the sequence illustrated in the accompanying equations for interrelating the stereochemistry of alcohols and carboxylic acids.
16,17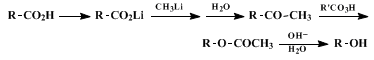
The reaction is believed to proceed by the indicated conversion of a
lithium carboxylate to the dilithium salt
18 which is stable at room temperature in the absence of proton-donating solvents or reactants. However, the rapidity with which this dilithium salt is decomposed to a ketone in the presence of proton donors, accompanied by the rapidity of the subsequent reaction of the ketone with more
methyllithium, can lead to a common side reaction in which an alkyldimethyl carbinol is formed. Both of the aforementioned reactions appear to be fast compared with 0.01 second usually required to disperse components with the conventional mixing techniques. This side reaction can be almost entirely avoided by taking precautions to minimize the possibility that high
local concentrations of the geminal dialkoxide and the
lithium reagent are present when a proton donor is added during either the reaction or the
subsequent quenching. It is frequently possible to obtain only relatively small amounts of the alcohol by-product by the dropwise addition
with vigorous mixing of 2 equivalents of the organolithium reagent
to a solution of the carboxylic acid. During this procedure it is not uncommon for the
lithium carboxylate to separate during the addition of the first mole of organolithium reagent and then to react and redissolve as the second equivalent of organolithium reagent is added. The reverse procedure, adding the acid to a solution of the organolithium reagent, appears always to produce substantial amounts of the alcohol by-product. The procedure used in this preparation illustrates how this mixing problem may be avoided by the initial conversion of the carboxylic acid to its lithium salt with
lithium hydride. In all cases it is important not to add a large excess of organolithium reagent and to add the final reaction mixture to the aqueous quenching bath slowly and with vigorous stirring if the formation of substantial amounts of alcohol by-product is to be avoided.
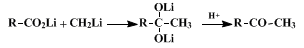
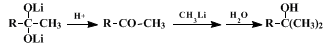
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
hydrochloric acid (7647-01-0)
ether (60-29-7)
acetic anhydride (108-24-7)
hydrogen (1333-74-0)
acetyl chloride (75-36-5)
Cyclohexanone (108-94-1)
Cyclohexene (110-83-8)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
cyclohexane (110-82-7)
methylmagnesium iodide (917-64-6)
Cyclohexanecarboxylic acid (98-89-5)
lithium (7439-93-2)
magnesium sulfate (7487-88-9)
1-Acetylcyclohexene (932-66-1)
lithium aluminum hydride (16853-85-3)
Methyllithium (917-54-4)
cyclohexanecarboxylic acid chloride (2719-27-9)
1,2-dimethoxyethane (110-71-4)
phenanthroline
sec-butyl alcohol (78-92-2)
Cyclohexyl methyl ketone,
Ketone, cyclohexyl methyl (823-76-7)
lithium hydride (7580-67-8)
lithium cyclohexanecarboxylate
bipyridyl (366-18-7)
lithium hydroxide (1310-65-2)
cyclohexyldimethylcarbinol
cyclohexylmethylcarbinol (4442-79-9)
lithium carboxylate
t-butyl α-chloropropionate (40058-88-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved