Org. Synth. 1921, 1, 75
DOI: 10.15227/orgsyn.001.0075
TRIMETHYLAMINE
Submitted by Roger Adams and B. K. Brown.
Checked by J. B. Conant and A. McB. Kinney.
1. Procedure
Five hundred grams (9.35 moles) of technical ammonium chloride and
1330 g. (14.8 moles) of paraformaldehyde are mixed and allowed to react as in the procedure described for the preparation of
trimethylamine hydrochloride (p. 531). To obtain from this crude reaction mixture a
water or alcohol solution of trimethylamine, it is not satisfactory merely to treat with alkali and lead the
trimethylamine through the solvent, since the gas is inefficiently absorbed; in fact, it is almost impossible to get a concentrated solution in this way without loss of material. The best way to prepare a solution is to liquefy the
trimethylamine (b.p.
3.5°) and in this form run it into the solvent. For this purpose, the
5-l. flask in which the initial reaction is carried out is fitted with a
rubber stopper holding a
separatory funnel and a
glass tube for leading off the
trimethylamine (Note 1). This tube is run into one opening of a
large U-tube or Wolff bottle holding
soda-lime for drying the gas. The exit tube from this drying bottle has a glass tube leading to the top of an
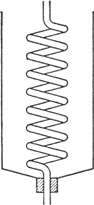
upright condenser which consists of a spiral tube cooled with a freezing mixture (Fig. 29)
(Note 2).
Fig. 29.
 |
The bottom of this condenser leads through a rubber stopper fitting tightly in a
2- or 3-l. flask, holding the solvent and immersed in an ice-salt mixture. An exit tube from this flask leads to a Wolff bottle kept at a low temperature and holding a little solvent, and through this solvent any unabsorbed
trimethylamine must bubble. The exit tube of this last Wolff bottle is closed with a
soda-lime tube if an anhydrous solvent is being used
(Note 3).
The separatory funnel is filled with sodium hydroxide solution (1100 g. in 2 l. of water) which is then allowed to flow into the reaction mixture at such a speed that, after the air in the apparatus has been replaced by trimethylamine, a continuous stream of drops of trimethylamine flows from the condenser into the solvent and practically no gas goes through the end Wolff bottle. At the beginning, the evolution of trimethylamine is rapid, and hence the addition of the alkali must be very slow (Note 4); as soon as the reaction mixture is partly decomposed, however, the alkali may be added more rapidly. After it has all been added, the reaction mixture is heated ten to fifteen minutes to make sure that all the amine is evolved. The time for this alkali treatment is about three to four hours.
With such an experiment as is described above, using
1000 g. of absolute alcohol (p. 249) in the solvent flask and about 150 g. in the end Wolff bottle,
1469 g. of solution is obtained in the main flask and
174 g. in the Wolff bottle, thus indicating that a total of
491 g. (
89 per cent of the theoretical amount) of
trimethylamine is present. There is thus obtained a
32 per cent solution in the main flask, which has a specific gravity of about 0.760 at +5°
(Note 5). By titration with standard
hydrochloric acid (Note 6), the same amounts of
trimethylamine are indicated as are found by weighing. In several experiments, the yields were consistently
85–90 per cent of the theoretical amount
(Note 7).
2. Notes
1.
As
trimethylamine is very volatile and easily lost, particular care must be taken to have all connections in the apparatus tight.
2.
Special attention should be drawn to the condenser (Fig. 29) used since it is valuable in many other experiments wherever substances having a boiling point between −10° and +30° are being handled (see, also,
p. 117). Any
gallon (4-l.) can, having an opening in the top in which a stopper may be inserted, is satisfactory for a jacket. The bottom of the can is cut out, and the can is then inverted. A spiral tube such as that which may be obtained from the inside of a
spiral condenser is placed in the can with the lower end fitting tightly through the stopper in the neck. By filling the can with an ice and salt mixture a condensing temperature of −10° or below can easily be obtained.
3.
If water is to be used as a solvent, no soda-lime tube is necessary on the exit of the end Wolff bottle. Moreover, with water as a solvent it is not absolutely necessary to have the soda-lime tube between the generating flask and the solvent. It is advisable to have it there, however, since it tends to catch any small amount of colored liquid coming from the reaction flask and thus prevents contamination of the water solution obtained.
4.
During the evolution of the
trimethylamine, it is necessary occasionally to shake the flask containing the solvent. It happens that, after about a
10–15 per cent solution is formed, the
trimethylamine tends to form a top layer which is not dissolved until agitated.
5.
In order to avoid loss of material, the specific gravity of any
trimethylamine solution must be taken at a low temperature. In this experiment, +5° was chosen.
6.
Titration of the strength of the
trimethylamine in the solvent is best done by pipetting out 1 cc. of the solution and allowing it to run immediately into 50 cc. of water. This is to prevent volatilization during titration. The amine is then titrated in the usual way with standard
hydrochloric acid, using
methyl orange as an indicator.
7.
Since tests in the
trimethylamine hydrochloride preparation
(p. 531) indicate that only
trimethylamine is formed in this reaction, it is unnecessary to purify the
trimethylamine solution.
3. Discussion
Trimethylamine can be prepared by the action of
formaldehyde on
ammonium chloride under pressure;
1 by the action of
formaldehyde and
formic acid on
ammonia,
2 a method which has been checked by one of the editors and is highly recommended; from
paraformaldehyde and
ammonium chloride;
3 and from
methyl alcohol, ammonia, and a catalyst.
4 The numerous commercial processes which lead to mixtures of methylamines have not been reviewed since they are not convenient for the laboratory.
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
soda-lime
alcohol (64-17-5)
hydrochloric acid (7647-01-0)
ammonia (7664-41-7)
methyl alcohol (67-56-1)
ammonium chloride (12125-02-9)
sodium hydroxide (1310-73-2)
formaldehyde (50-00-0)
formic acid (64-18-6)
Trimethylamine (75-50-3)
Trimethylamine hydrochloride (593-81-7)
methyl orange (547-58-0)
paraformaldehyde (30525-89-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved