Org. Synth. 1943, 23, 8
DOI: 10.15227/orgsyn.023.0008
BROMOACETAL
[Acetaldehyde, bromo-, diethyl acetal]
Submitted by S. M. McElvain and D. Kundiger.
Checked by R. L. Shriner and C. H. Tilford.
1. Procedure
An apparatus is assembled as shown in
Fig. 5. The
3-l. three-necked round-bottomed flask A is equipped with a mechanical stirrer sealed with a well-lubricated rubber sleeve. In one neck of the flask are fitted a
thermometer and a
glass tube leading through a safety trap B to a water pump. In the other neck a
7-mm. glass tube, extending to the bottom of the flask, is attached to a
500-ml. bottle C in which is placed
255 ml. (5 moles) of bromine (Note 1). This bottle is connected to a
500-ml. wash bottle D containing
250 ml. of sulfuric acid. The
inlet tube of D is connected to a
calcium chloride tube.
Fig. 5.
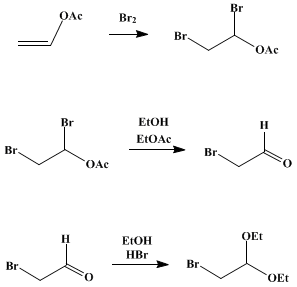
A solution of 430 g. (5 moles) of vinyl acetate (Note 2) in 1.5 l. (26 moles) of absolute ethanol is placed in flask A. The solution is cooled to about −10° by an ice-salt mixture, and stirring is started. Gentle suction is applied at the outlet tube of B, and the bromine is introduced into A by a rapid current of air. The rate of introduction of the bromine, controlled by adjustment of the clamp E, should be regulated so that 8–10 hours is required to volatilize all the bromine. Stirring is stopped, and the reaction mixture is allowed to stand overnight and to come to the temperature of the room. The mixture is poured into 1.7 l. of ice water (Note 3); the lower layer of bromoacetal and ethyl acetate is separated (Note 4), washed twice with 300-ml. portions of cold water and once with 300 ml. of cold 10% sodium carbonate solution, and dried over two successive 25-g. portions of anhydrous calcium chloride for 30 minutes. The crude product weighs 990–1010 g.; it is purified by distillation under diminished pressure (water pump) through a 6-in. Widmer column or a 20-cm. Vigreux column. The first fraction consists of ethyl acetate; this is followed by the pure bromoacetal which boils at 62–63°/15 mm. (84–85°/30 mm.) and which amounts to 610–625 g. (62–64%) (Note 5).
2. Notes
1.
The
bromine is previously washed with
100 ml. of concentrated sulfuric acid.
2.
The
vinyl acetate (Eastman Kodak Company) is distilled from the added preservative; the first turbid portion containing water is discarded, and the fraction boiling at
69–71°/740 mm. is used.
3.
If an emulsion forms at this point, 320 g. of hydrated
sodium sulfate is added.
4.
Bromoacetal is a fairly strong lachrymator and is best handled under a
hood.
5.
A yield of
78% of
bromoacetal has been obtained by a slight modification of the procedure described above. One-half of the amounts of materials specified above were used, and, after the bromination was complete, the reaction mixture was allowed to stand for 64 hours before it was processed. The yield of
bromoacetal was
380 g.1
3. Discussion
The procedure given above is essentially a large-scale adaptation of that of Filachione.
2 Bromoacetal has been prepared by the bromination of
acetal directly,
3 or in the presence of
calcium carbonate;
4 by action of
sodium ethoxide on
α,β-dibromodiethyl ether;
5 by bromination of
paraldehyde followed by action of
ethanol;
6,7 by the action of
ethanol on
bromoacetaldehyde;
8,9 and by bromination of
acetal with
N-bromosuccinimide.
10This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Bromoacetal
ACETAL (105-57-7)
ethanol (64-17-5)
calcium chloride (10043-52-4)
sulfuric acid (7664-93-9)
ethyl acetate (141-78-6)
sodium carbonate (497-19-8)
bromine (7726-95-6)
sodium sulfate (7757-82-6)
calcium carbonate (471-34-1)
sodium ethoxide (141-52-6)
N-bromosuccinimide (128-08-5)
Acetaldehyde, bromo-, diethyl acetal
vinyl acetate (108-05-4)
α,β-dibromodiethyl ether (2983-26-8)
bromoacetaldehyde (17157-48-1)
paraldehyde (123-53-7)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved