Org. Synth. 1979, 59, 1
DOI: 10.15227/orgsyn.059.0001
1-N-ACYLAMINO-1,3-DIENES FROM 2,4-PENTADIENOIC ACIDS BY THE CURTIUS REARRANGEMENT: BENZYL trans-1,3-BUTADIENE-1-CARBAMATE
[Carbamic acid, 1,3-butadienyl-, (E)-, phenylmethyl ester]
Submitted by Peter J. Jessup, C. Bruce Petty, Jan Roos, and Larry E. Overman
1.
Checked by Paul H. Liang and G. Büchi.
1. Procedure
A. trans-2,4-Pentadienoic acid. A 1-l., three-necked, round-bottomed flask is equipped with a mechanical stirrer, a condenser cooled with ice-cold water (Note 1) bearing a calcium chloride drying tube, and a powder funnel. The flask is charged with 206 g. (210 ml., 2.61 moles) of pyridine (Note 2), and with vigorous stirring 208 g. (2.00 moles) of powdered malonic acid (Note 3) is added in portions. The powder funnel is replaced by a 250-ml., pressure-equalizing dropping funnel containing 126 g. (150 ml., 2.25 moles) of acrolein (Note 4), which is added, with vigorous stirring, over a 30-minute period. The exothermic reaction begins immediately with evolution of carbon dioxide, and the gently refluxing mixture becomes homogeneous. After 1 hour, as carbon dioxide evolution ceases, the solution is poured onto 1 l. of ice in a 3-l. Erlenmeyer flask, and carefully acidified with 130 ml. of concentrated sulfuric acid. The aqueous layer is extracted with four 250-ml. portions of dichloromethane, and the organic extracts are dried over magnesium sulfate for ca. 10 minutes. The dichloromethane solution is concentrated to ca. 300 ml. on a rotary evaporator with the water bath at 20–30° and allowed to crystallize in a freezer at −10° for several hours. After a first crop, yielding 40–50 g. of product, m.p. 72–73°, is collected, three additional crops are taken following successive concentration of the mother liquor to 150, 70, and 30 ml. Dried under reduced pressure in the presence of phosphorous pentoxide, the four crops of off-white crystals total 82–90 g. (42–46%), m.p. 69–71° (Note 5).
B. Benzyl trans-1,3-butadiene-1-carbamate. Caution! The following reaction should be carried out in a fume hood to avoid accidental exposure to toxic hydrazoic acid.
A dry, 1-l., three-necked, round-bottomed flask is equipped with a magnetic stirring bar, a thermometer, and a 250-ml., pressure-equalizing dropping funnel bearing a nitrogen inlet. The flask is flushed with nitrogen and charged with 49 g. (0.50 mole) of trans-2,4-pentadienoic acid, 80 g. (0.62 mole) of N,N-diisopropylethylamine, and 300 ml. of acetone (Note 6). The resulting solution is stirred and cooled to 0° in an ice–salt bath. A solution of 55 g. (0.51 mole) of ethyl chloroformate in 150 ml. of acetone is added over 30 minutes while the temperature is maintained below 0° (Note 7). Stirring is continued for an additional 30 minutes at 0°, after which a chilled solution of 65 g. (1.0 mole) of sodium azide (Note 8) in 170 ml. of water is added over a 20-minute interval, keeping the temperature below 0°. The contents of the flask are stirred for an additional 10–15 minutes at 0° (Note 9) and poured into a 2-l. separatory funnel containing 500 ml. of ice-water. The acyl azide is isolated by extraction with six 250-ml. portions of toluene. The combined toluene extracts are dried over anhydrous magnesium sulfate for 20 minutes and concentrated to a volume of ca. 300 ml. on a rotary evaporator at a water bath temperature of 40–50° (Note 10). Caution! The acyl azide is potentially explosive. The solution should not be evaporated to dryness. While the toluene solution is being concentrated, a dry, 2-l., three-necked, round-bottomed flask equipped with a mechanical stirrer, a 500-ml. pressure-equalizing dropping funnel, a simple distillation head, and a heating mantle is charged with 43 g. (0.40 mole) of benzyl alcohol, 250 mg. of 4-tert-butylcatechol (Note 11), and 200 ml. of toluene. About 30 ml. of toulene is distilled from the flask to remove trace amounts of water, and the distillation head is replaced with a condenser fitted with a nitrogen inlet. The toluene solution is stirred and heated at a rapid reflux under a nitrogen atmosphere as the toluene solution of the acyl azide is added over 30 minutes. The disappearance of the acyl azide and isocyanate is followed by IR analysis (Note 12). Conversion to the carbamate is complete in 10–30 minutes, after which the solution is cooled rapidly to room temperature by immersing the flask in an ice bath. The toluene is rapidly removed on a rotary evaporator with the water bath at 40–50°, producing a yellow solid residue (Note 13) which is dissolved in 50 ml. of 95% ethanol and allowed to crystallize in a freezer at −25° for several hours. Two crops of pale yellow crystals, m.p. 69–72°, are isolated which total 39–46 g. after drying under reduced pressure. Concentration of the mother liquor affords an oily residue that is placed on a 6 × 80-cm. column packed with 500 g. of silica gel (Note 14) and eluted with 1:9 (v/v) ethyl acetate–hexane. An additional 11–12 g. of crystalline product is obtained from the chromatography, raising the total yield to 50–58 g. (49–57%) of nearly pure benzyl trans-1,3-butadiene-1-carbamate, a pale yellow solid, m.p. 70–73° (Note 15).
2. Notes
1.
A
water pump purchased from Little Giant Pump Company, Oklahoma City, Oklahoma, was used by the submitters to circulate ice-water through the condenser. The checkers used a
dry ice condenser.
2.
Reagent grade pyridine was stored over Linde type 4A molecular sieves for at least 24 hours before use.
3.
Malonic acid was purchased from Aldrich Chemical Company, Inc. Most, but not all, of the
malonic acid dissolves after 30 minutes. If
pyridine is added to the
malonic acid, a hard cake results.
4.
Acrolein contaminated by 3% water was obtained by the submitters from Aldrich Chemical Company, Inc., and was purified by distillation from
anhydrous calcium sulfate, b.p.
53°. The checkers used
acrolein purchased from MC and B Manufacturing Chemists.
5.
This material is of satisfactory purity for use in Part B. A thin-layer chromatogram on silica gel developed with 1:1 (v/v)
ethyl acetate–hexane containing 1% acetic acid and visualized with 10% phosphomolybdic acid in ethanol as a spray reagent showed a major spot at
Rf = 0.4 and a faint spot at 0.2. The crystalline acid may be stored for several months without significant decomposition. A melting point of
72° is reported
2 for
trans-2,4-pentadienoic acid. The spectral properties of the product are as follows: IR (CHCl
3) cm.
−1: 3200–2700 (OH), 1696 (C=O), 1640, 1600, 1280, 1010;
1H NMR (CDCl
3), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 5.1–5.8 (m, 2H, two
H at C-5), 5.92 (d,
J = 15, 1H,
H at C-2), 6.2–6.8 (m, 1H,
H at C-4), 7.37 (d of d,
J2,3 = 15,
J3,4 = 11, 1H,
H at C-3), 12.0 (s, 1H, CO
2H).
6.
N,N-Diisopropylethylamine was supplied by Aldrich Chemical Company, Inc., and purified by distillation from
sodium hydride, b.p.
127°.
Reagent grade acetone was stored over Linde type 4A molecular sieves for at least 24 hours before use.
7.
Ethyl chloroformate obtained from Aldrich Chemical Company, Inc., was distilled, b.p.
93°. The progress of the reaction may be followed by
1H NMR spectroscopy. Aliquots are partitioned between
dichloromethane and water, the organic layer is concentrated, and the spectrum is recorded. A quartet from the ethoxy group of the mixed anhydride appears at δ 4.2.
Ethyl chloroformate, which exhibits a quartet at δ 4.3, is removed in the concentration step.
8.
Analytical reagent grade sodium azide purchased from Alfa Division, Ventron Corporation, or J. T. Baker Chemical Company was used as supplied.
9.
The formation of the acyl azide may be followed by the growth of the 2130-cm.
−1 (-N=N=N) IR absorption of concentrated
dichloromethane extracts of aliquots removed from the reaction.
10.
The solution is concentrated for the purpose of removing residual
ethanol. If this step is omitted,
ethyl trans-1,3-butadiene-1-carbamate will be formed, contaminating the final product.
11.
Reagent grade benzyl alcohol was purified by distillation, b.p.
205°.
4-tert-Butylcatechol purchased from Aldrich Chemical Company was sublimed at 50° (0.1 mm.) and recrystallized from
hexane.
12.
The acyl azide IR absorption occurs at 2130 cm.
−1 and the
isocyanate at 2270 cm.
−1.
13.
Impure samples of the product are particularly prone to decomposition. The purification steps should be carried out immediately.
14.
Grade 60, activity III silica gel, purchased from W. R. Grace and Company, was used.
15.
Material of this purity is suitable for most applications and may be stored in a freezer for several months with only slight decomposition. A thin-layer chromatogram on silica gel developed with
1:3 (v/v) ethyl acetate–hexane and visualized by UV fluorescence showed a single spot.
Benzyl trans-1,3-butadiene-1-carbamate, like all
N-acylamino-1,3-dienes, is quite acid sensitive and can, for example, be decomposed by traces of
deuterium chloride in
chloroform-d. A pure sample may be obtained by recrystallization from
1:20 (v/v) ethyl acetate–hexane, m.p.
74–75°; IR (Nujol) cm.
−1: 3300, 1692, 1625, 1515, 1230, 690;
1H NMR (CDCl
3), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 4.8–5.0 (m, 2H, two
H at C-4), 5.15 (s, 2H, C
H2C
6H
5), 5.4–5.8 (m, 2H,
H at C-2 and N
H), 6.26 (apparent d of t,
J = 10 and
J = 17, 1H,
H at C-3), 6.71 (broadened d,
J = 9, 1H,
H at C-1), 7.33 (s, 5H, C
6H5);
13C NMR (CDCl
3), δ (assignment): 67.5 (
CH
2C
6H
5), 112.5 (
C-2), 113.5 (
C-4), 127.2 (
C-1), 128.3 (
para-C
6H
5), 128.4 (
meta-C
6H
5), 128.7 (
ortho-C
6H
5), 134.6 (
C-3), 136.0 (
peri-C
6H
5), 153.7 (
C=O).
3. Discussion
This procedure illustrates a general method
3 for the preparation of
trans-1-
N-acylamino-1,3-butadienes from 2,4-pentadienoic acids. A number of 1,3-butadienyl and 1,3-pentadienyl carbamates, thiocarbamates, and ureas have been prepared by this procedure (Table I).
3 These dienamides are reasonably stable crystalline solids which, when pure, may be stored in a freezer for several months with little decomposition. Since a variety of conjugated dienoic acids are readily accessible from Knoevenagel, Wittig, and related reactions,
4 1-
N-acylamino-1,3-butadienes with a diversity of carbon skeletons and heteroatom acyl substituents are potentially available by this method. Although this procedure describes the only known preparation of
benzyl trans-1,3-butadiene-1-carbamate, a number of similar 1-
N-acylamino-1,3-butadiene derivatives have been prepared by Curtius rearrangement of
2,4-hexadienoyl azide,
5 by Hofmann rearrangement of
2,4-hexadienamide,
6 by
N-acylation of 1-
N-alkylamino-1,3-butadiene anions generated from crotonaldehyde imines,
7 and by thermal rearrangement of trichloroacetimidic esters of acetylenic alcohols.
8The Curtius rearrangement procedure described here is a modification of one reported by Weinstock.
9 The submitters have found this procedure to be considerably more reproducible when
N,N-diisopropylethylamine is substituted for
triethylamine. The procedure described for the preparation of
trans-2,4-pentadienoic acid is a modification of an earlier one by Doebner.
10 The submitters have found this method to give reproducibly higher yields, and to be more convenient, than other commonly used procedures for preparing this material.
2,11 The use of
dichloromethane as the extracting and crystallizing solvent greatly simplifies the isolation of polymer-free samples of the crystalline acid.
trans-1-
N-Acylamino-1,3-butadienes are useful dienes in Diels-Alder reactions. They are the most convenient synthetic equivalents currently available for the parent 1-amino-1,3-butadienes. These electron-rich dienamides undergo Diels-Alder cycloaddition with remarkable ease and high regio- and stereoselectivity.
12,13 As illustrated below, they react readily with even relatively unreactive dienophiles such as
methyl acrylate and
trans-crotonaldehyde. A recent quantitative study
12 has confirmed the expectation of an increase in reactivity with increasing electron-donating ability of the acyl substituent of an acylaminobutadiene, although the effects observed are not large.
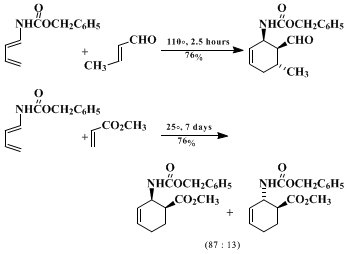
The choice of the acyl substituent X for Diels-Alder reactions of 1-
N-acylamino-1,3-butadienes depends on the particular synthetic problem. The acyl substituent has a moderate effect on the cycloaddition reactivity of these dienes,
12 and also determines what amine unmasking procedures are required. As a result of their stability
12,13 and the variety of amine deprotection procedures available,
14 the diene carbamates are the components of choice in most cases. A particularly attractive aspect of the diene synthesis detailed here is the ability to "tailor" the amino-protecting group (
) for the application at hand.
Benzyl trans-1,3-butadiene-1-carbamate has the amino group protected with the benzyloxycarbonyl group. The ability to remove this functionality by catalytic hydrogenation was an important design feature in a recent alkaloid synthesis
13 that utilized this diene.
TABLE I
PREPARATION OF trans-1-N-ACYLAMINO-1,3-BUTADIENES3
|
|
|
|
R
|
X
|
Procedurea
|
M.p. (°)
|
Yield (%)
|
|
|
H
|
OCH2C6H5
|
A
|
74–75
|
53b
|
|
H
|
OC(CH3)3
|
A
|
67–68
|
59
|
|
CH3
|
OCH2CH3
|
A
|
91–92
|
80
|
|
H
|
OCH2CH3
|
A
|
44–45
|
71b
|
|
CH3
|
OC6H5
|
A
|
118–120
|
72
|
|
H
|
OC6H5
|
Bc
|
118–119
|
66
|
|
A
|
|
45
|
|
CH3
|
N(CH2)4
|
B
|
164–165
|
77
|
|
A
|
|
(10)d
|
|
H
|
N(CH2)4
|
B
|
163–164
|
44
|
|
CH3
|
SC6H5
|
B
|
116–118
|
78
|
|
H
|
SC6H5
|
B
|
92–93
|
47
|
|
aIsocyanate trapped as formed at 100° (Procedure A) or isocyanate preformed at 110° and trapped at 25° (Procedure B).
|
bMean yield of four runs. All other entries are non-optimized yields from one run.
|
cA few drops of triethylamine were added.
|
dEstimated from the 1H NMR spectrum.
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
phosphorous pentoxide
ethanol (64-17-5)
sulfuric acid (7664-93-9)
acetic acid (64-19-7)
ethyl acetate (141-78-6)
Acrolein (107-02-8)
nitrogen (7727-37-9)
carbon dioxide (124-38-9)
calcium sulfate (7778-18-9)
acetone (67-64-1)
pyridine (110-86-1)
toluene (108-88-3)
Benzyl alcohol (100-51-6)
sodium azide (26628-22-8)
dichloromethane (75-09-2)
Malonic acid (141-82-2)
ethyl chloroformate (541-41-3)
methyl acrylate (96-33-3)
magnesium sulfate (7487-88-9)
isocyanate
sodium hydride (7646-69-7)
hexane (110-54-3)
trans-crotonaldehyde (123-73-9)
triethylamine (121-44-8)
chloroform-d (865-49-6)
phosphomolybdic acid (51429-74-4)
deuterium chloride (7698-05-7)
2,4-hexadienoyl azide
2,4-hexadienamide
N,N-diisopropylethylamine (7087-68-5)
4-tert-butylcatechol (98-29-3)
Benzyl trans-1,3-butadiene-1-carbamate,
Carbamic acid, 1,3-butadienyl-, (E)-, phenylmethyl ester (71616-72-3)
trans-2,4-Pentadienoic acid (21651-12-7)
ethyl trans-1,3-butadiene-1-carbamate
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved