Org. Synth. 1977, 57, 26
DOI: 10.15227/orgsyn.057.0026
(Z)-4-CHLORO-4-HEXENYL TRIFLUOROACETATE
[Acetic acid, trifluoro-, (Z)-4-chloro-4-hexenyl ester]
Submitted by P. E. Peterson and M. Dunham
1.
Checked by K.-C. Luk and G. Büchi.
1. Procedure
A. 6-Chloro-2-hexyne. A 2-l., three-necked, round-bottomed flask is equipped with a low-temperature condenser (Note 1), a gas-inlet, and a magnetic stirrer. Sodium hydroxide drying tubes are placed to precede the inlet and on the condenser. The system is purged with nitrogen, and approximately 650 ml. of anhydrous ammonia (Note 2) is condensed. Freshly cut sodium metal (20.2 g., 0.878 g.-atom) is added to the refluxing ammonia. After dissolution of the sodium (about 20 minutes is required), 0.2 g. of iron(III) nitrate is added. Two hours of stirring are allowed for conversion of the deep-blue solution of sodium in ammonia to sodium amide. The reaction mixture is cooled to −73° using an acetone-dry ice bath and 35.3 g. (50.0 ml., 0.882 mole) of precondensed propyne (Note 3), (Note 4), is added in portions over a 1-minute period through a glass funnel precooled in dry ice (Note 4). The mixture is stirred, with continued cooling for 15 minutes, and 153.5 g. (0.975 mole) of 1-bromo-3-chloropropane (Note 5) is added from an addition funnel over 20 minutes (Note 6). After 30 minutes of additional stirring, 250 ml. of diethyl ether is added to the flask, the dry ice bath is removed, and the ammonia is allowed to evaporate (Note 7). Water (200 ml.) is added to the reaction vessel, and the resulting solution is transferred to a 1-l. separatory funnel. The water layer is removed and extracted once with 100 ml. of ether. The combined ether extracts are treated with 6 M hydrochloric acid until the aqueous layer is acidic (approximately 20 ml. is required). The ether layer is separated and dried in three stages over magnesium sulfate. Removal of the solvent and distillation of the crude product through a 20-cm. Widmer column (Note 8) yields 49.4–56.0 g. of 6-chloro-2-hexyne, b.p. 58–64° (20 mm.), which contained some 1-bromo-3-chloropropane (to be removed in the next step). The corrected yield is 29–31% (Note 9).
B. 4-Chloro-4-hexenyl trifluoroacetate. A 500-ml., one-necked flask equipped with a magnetic stirrer is charged with 200 ml. of redistilled trifluoroacetic acid (Note 10) and 6-chloro-2-hexyne (0.16 mole, calculated from GC analysis) containing 1-bromo-3-chloropropane. The flask is fitted with a Friedrichs condenser, and the mixture is refluxed for 3 hours. The flask is transferred to a vacuum distillation apparatus; with the aid of an aspirator and a controlled leak, excess trifluoroacetic acid is removed under slightly reduced pressure, maintaining the pot temperature below 65° to prevent reactions of the acid with the double bond. The remaining trifluoroacetic acid is removed by pouring the product into 100 ml. of ice water and extracting with 100- and 50-ml. portions of dichloromethane. The organic extracts are brought to pH 7 with saturated sodium hydrogen carbonate (ca. 40 ml.). The resulting aqueous layer is extracted once with an additional 25 ml. of dichloromethane, and the combined dichloromethane extracts are dried in three stages by stirring over magnesium sulfate. The final stage is allowed to stand in a refrigerator for 24 hours. The solvent is removed by distillation through a 20-cm. Widmer column ((Note 8)) at atmospheric pressure. Distillation at reduced pressure gives 16.0–16.4 g. of 4-chloro-4-hexenyl trifluoroacetate, b.p. 86–89° (22 mm.) (Note 11), which contains trace amounts of 1-bromo-3-chloropropane (Note 12). On the basis of the alkyne present in the reactant the yield is 50–52%.
2. Notes
1.
A
cold finger condenser packed with dry ice and
2-propanol may be used. A Friedrichs condenser in combination with a circulating low-temperature bath (−70°) is more convenient.
2.
Ammonia was purchased from Matheson Gas Products.
3.
Propyne can be purchased from Linde Specialty Gases or Farchan Research Laboratories. Matheson Gas Products sells
propyne also, but only in 100-lb. quantities.
4.
The
propyne (b.p.
−23.2°) is precondensed to the mark in a volumetric flask cooled by acetone–dry ice. Evaporation of some
propyne during addition will lead to a moderate molar excess of
1-bromo-3-chloropropane, regarded as desirable in preventing formation of diyne product.
5.
1-Bromo-3-chloropropane was purchased from Aldrich Chemical Company, Inc.6.
Sudden foaming occurred in a run involving insufficient cooling or overly rapid additions. Slow addition could lead to diyne product.
7.
The checkers maintained the condenser at −5° during the evaporation to minimize loss of alkyne.
8.
The submitters use a
platinum spinning band apparatus for the distillation.
9.
GC analysis at 79° using a flame detector in conjunction with a
183 × 0.32 cm. stainless-steel column containing Dow-Corning 550 fluid on silanized support gave peaks for
1-bromo-3-chloropropane (6.5 minutes) and
6-chloro-2-hexyne (9.3 minutes) whose areas were shown to be proportional to the mole fractions. The latter were determined by integration of the expanded (50 Hz. sweep width) 100 MHz.
1H NMR spectrum in the region of overlapping triplets near δ 3.6.
10.
The checkers purchased
trifluoroacetic acid from Aldrich Chemical Company, Inc., and distilled it from
phosphorous pentoxide. The submitters point out that some
trifluoroacetic anhydride, whose effects have not been fully investigated, is obtained under these conditions. The submitters prefer to use
trifluoroacetic acid which has been distilled through a glass packed column without the use of a drying agent.
11.
nD25 1.4025;
1H NMR (CCl
4), δ (multiplicity, coupling constant
J in Hz., number of protons): 1.70 (d,
J = 7, 3H), 1.8–2.6 (m, 4H), 4.35 (t,
J = 6, 2H), 5.58 (q,
J = 7, 1H).
12.
1-Bromo-3-chloropropane, b.p.
46–55° (15 mm.) is well separated in the early fractions. GC analysis at 120° (
cf. (Note 9)) gives peaks of proportional areas for
1-bromo-3-chlororopane (1.2 minutes),
4-chloro-4-hexenyl trifluoroacetate (2.3 minutes), and traces of
4-chloro-4-hexen-1-ol (2.5 minutes).
3. Discussion
The presence, in the
4-chloro-4-hexenyl trifluoroacetate, of small amounts of two
cis–trans pairs of products of addition of
trifluoroacetic acid to the triple bond without concomitant halogen shift remains speculative. In any event, these compounds would be removed as ketones upon hydrolysis
2 of the
trifluoroacetate. Both the
4-chloro-4-hexenyl trifluoroacetate and the alcohol resulting from its hydrolysis have been shown to contain 9% of the (
E) isomer.
2 In the present study, the
hydrogen decoupled
13C magnetic resonance spectra of the ester and alcohol were shown to contain peaks attributable to approximately 9% of (
E) isomer.
Unsymmetrical
trans-vinyl halides have been prepared from acetylenic alcohols by Corey and co-workers
3 4 (as illustrated in the accompanying formulation) in connection with their synthesis of
farnesol and
Cecropia juvenile hormone. Several syntheses of vinyl halides (with identical R groups,
trans) have been reported, including procedures involving halogenation and elimination,
5 addition of
hydrochloric acid to alkynes,
6 and preparation from alkynes
via vinyl alanes.
7 The synthesis of unsymmetrical
trans-vinyl halides by 1,4-halogen shift reactions is exemplified by the procedure given here. Such compounds are potentially useful in the synthesis of compounds containing trisubstituted double bonds.
8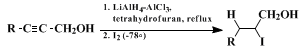
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
phosphorous pentoxide
1-bromo-3-chlororopane
hydrochloric acid (7647-01-0)
ammonia (7664-41-7)
ether,
diethyl ether (60-29-7)
hydrogen (1333-74-0)
sodium hydrogen carbonate (144-55-8)
nitrogen (7727-37-9)
sodium (13966-32-0)
2-propanol (67-63-0)
1-bromo-3-chloropropane (109-70-6)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
sodium amide (7782-92-5)
iron(III) nitrate
trifluoroacetic acid (76-05-1)
propyne (74-99-7)
trifluoroacetic anhydride (407-25-0)
6-chloro-2-hexyne (28077-73-8)
4-chloro-4-hexenyl trifluoroacetate
4-chloro-4-hexen-1-ol
trifluoroacetate
farnesol
(Z)-4-Chloro-4-hexenyl trifluoroacetate,
Acetic acid, trifluoro-, (Z)-4-chloro-4-hexenyl ester (28077-77-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved