Org. Synth. 1974, 54, 27
DOI: 10.15227/orgsyn.054.0027
CYCLOPROPYLDIPHENYLSULFONIUM TETRAFLUOROBORATE
[Sulfonium, cyclopropyldiphenyl tetrafluoroborate(1-)]
Submitted by Mitchell J. Bogdanowicz and Barry M. Trost
1.
Checked by Tsutomu Aoki and Wataru Nagata.
1. Procedure
A. 3-Chloropropyldiphenylsulfonium tetrafluoroborate. A solution of 93.0 g. (0.500 mole) of diphenyl sulfide (Note 1) and (Note 2) and 347 g. (1.70 mole) of 1-chloro-3-iodopropane (Note 2), (Note 3), and (Note 4) in 200 ml. of nitromethane (Note 5) in a 1-l., one-necked flask equipped with a magnetic stirring bar and a nitrogen inlet tube is stirred at room temperature under nitrogen. The flask is shielded from light (Note 6), and 78 g. (0.40 mole) of silver tetrafluoroborate (Note 7) is added in one portion. Initially the temperature rises to 40°, then gradually falls to room temperature. No external cooling is necessary. After 16 hours 200 ml. of dichloromethane is added, the mixture is filtered through a sintered glass funnel fitted with a 35 g. pad of Florisil (Note 8), and the solid is washed with 100 ml. of dichloromethane. The dichloromethane portions are combined and evaporated at reduced pressure until a solid separates; 1 l. of diethyl ether is added to precipitate the product (Note 9). The off-white crystals are collected (Note 10), washed with ether, and dried under reduced pressure at 25°, yielding 122–140 g. (87–99%) of the sulfonium salt, m.p. 103–105° (Note 11).
B. Cyclopropyldiphenylsulfonium tetrafluoroborate. A suspension of 118.7 g. (0.3386 mole) of 3-chloropropyldiphenylsulfonium tetrafluoroborate (Note 2) in 500 ml. of dry tetrahydrofuran (Note 12) is placed in a 2-l., one-necked flask equipped with a magnetic stirring bar and nitrogen inlet tube under nitrogen before 5-g. portions of 55% sodium hydride–mineral oil dispersion (15.2 g., 0.350 mole) are added in 30-minute intervals. The resulting mixture is stirred (Note 13) at room temperature for 24 hours. An aqueous solution of 25 ml. of 48% fluoroboric acid (Note 14), 15 g. of sodium tetrafluoroborate (Note 7), (Note 15), and 400 ml. of water is added to the well-stirred reaction to destroy residual hydride and swamp out chloride ion (Note 16). After 5 minutes 300 ml. of dichloromethane is added, and the top organic layer is removed from the lower aqueous layer (Note 17). The dichloromethane solution is then extracted with 100 ml. of water. The combined water layers are extracted with an additional 100 ml. of dichloromethane. The organic phases are combined, dried over anhydrous sodium sulfate, and evaporated at reduced pressure until precipitation occurs. Addition of 1 l. of ether completes the precipitation of the salt. The crystals are collected, washed with ether, recrystallized from hot absolute ethanol (approximately 400 ml.) (Note 18), and dried under reduced pressure, yielding 79.5–88.0 g. (75–83%) of cyclopropyldiphenylsulfonium tetrafluoroborate, m.p. 137–139° (Note 19).
2. Notes
1.
Available from Matheson, Coleman and Bell and utilized without further purification. The checkers used
reagent grade diphenyl sulfide obtained from Tokyo Kasei Kogyo Co. Ltd., Japan.
2.
The checkers carried out the experiment on a half scale and obtained the same results as described by the submitters.
3.
Available from K & K Laboratories or may be prepared in
89% yield by the following procedure. To a solution of
393 g. (2.63 moles) of sodium iodide in
1 l. of reagent grade acetone is added
394 g. (2.50 moles) of 1-bromo-3-chloropropane (Aldrich Chemical Co.). After stirring 2 hours at room temperature, the mixture is filtered, the
sodium bromide is washed with
acetone, and the
acetone is evaporated at reduced pressure. A dark iodine color is present along with some solid sodium salts. The oil is dissolved in ether, and the solution is washed with a
10% aqueous sodium thiosulfate. The ethereal layer is separated, dried over anhydrous
sodium sulfate, and evaporated at reduced pressure, yielding
454 g. of an oil that can be used without further purification.
4.
An excess of
1-chloro-3-iodopropane must be employed to compete effectively with the
diphenyl sulfide for complexation with
silver fluoroborate.
5.
Available from Aldrich Chemical Co. and used without further purification.
Dichloromethane may be substituted for the
nitromethane. The checkers used
reagent grade nitromethane available from Tokyo Kasei Kogyo Co. Ltd., Japan.
6.
The flask is wrapped with
aluminium foil to prevent decomposition of the silver salts.
7.
Available from Ozark Mahoning Corp.
8.
A pad of Florisil is employed to facilitate removal of the suspended silver salts.
9.
An oil separated initially. Vigorous shaking of the mixture to extract the excess starting material out of the oily sulfonium salt layer induces crystallization.
10.
The crystals obtained by the checkers were light brown at this stage, but could be purified by the following procedure.
11.
The material is normally utilized directly without further purification. If the solid is very gray, it may be recrystallized. For recrystallization the salt is dissolved in hot
95% ethanol (approximately 350 ml. per 100 g. of salt) containing
decolorizing carbon and filtered rapidly. The clear supernatant liquid is allowed to cool in a freezer (−20°). In this way, white crystals, m.p.
106–107°, may be obtained with nearly quantitative recovery. The checkers obtained the purified material, m.p.
108–109°, with 95% recovery and used it for the next step. The purified material has the following spectral data; UV (95% C
2H
5OH) nm. max (ε): 236 shoulder (13,200), 262 (2200), 268 (2680), 275 (1010); IR (Nujol) cm.
−1: 3090 weak, 3060 weak (aromatic CH), 1580 weak (C=C);
1H NMR (CDCl
3), first-order analysis: δ 2–2.5 (m,
Jbc = 8 Hz.,
Jcd = 6.5 Hz., 2H, C
H2c). 3.75 (t, 2H, C
H2d), 4.3 (poorly resolved t, 2H, C
H2b), 7.5–8.2 (m, 10H, 2C
6H5a).
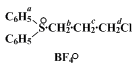
12.
The
tetrahydrofuran was dried by distilling from
lithium aluminium hydride and then from
sodium benzophenone ketyl (generated by adding small pieces of
sodium metal and
benzophenone) directly into the reaction flask. A blue-black color of the ketyl solution indicates dryness. The checkers purified
terahydrofuran by distillation from
sodium hydride dispersion under
nitrogen, and used it immediately.
13.
The checkers found that efficient stirring is essential for successful results.
14.
Since
48% fluoroboric acid was not available in Japan, the checkers used
42% fluoroboric acid obtained from Wako Pure Chemicals Co. Ltd., and obtained the same result as described by the submitters.
15.
The checkers prepared
sodium tetrafluoroborate by neutralization of an ice-cold, aqueous,
42% solution of fluoroboric acid with an equivalent amount of
sodium carbonate and addition of dry
ethanol to the reaction mixture to effect complete crystallization of the product. The crystals were purified by washing with
ethanol and obtained in
80% yield.
16.
Normally no observable effect occurs upon this addition. Gas evolution with a slight exotherm indicates incomplete reaction.
17.
The density of the
dichloromethane and water layers are nearly equal. Thus, sometimes upon initial mixing, the
dichloromethane starts out on the bottom, but the layers reverse on shaking. However, on occasion, the desired organic layer is found in fact to be the bottom one. It is therefore advisable to check the layers by addition of either water or
dichloromethane. The checkers found that
dichloromethane was on the bottom in the two experiments.
18.
Ether may be added to the cold
ethanol solution before filtration to insure complete precipitation.
19.
The purified material has the following spectral data; UV (95% C
2H
5OH) nm. max (ε): 235 shoulder (12,200), 261 (1800), 267 (2200), 274 (1700); IR (Nujol) cm
−1: 3100 weak, 3045 weak (aromatic CH), 1582 weak (C=C);
1H NMR (CDCl
3), first-order analysis: δ 1.4–1.75 (m, 4H, 2C
H2c), 3.5–3.9 (m, 1H, C
Hb), 7.5–8.1 (m, 10H, 2C
6H5a).
3. Discussion
The utility of sulfur ylides in organic synthesis demands methods for the efficient preparation of the precursor
sulfonium salts.
2 Among the salts, the
diphenylsulfonium moiety provides the ability to generate the higher alkylides unambiguously but the low nucleophilicity of the
sulfur of
diphenyl sulfide dictated the need for exceptionally reactive alkylating agents. Oxonium salts,
3 dialkoxycarbonium salts,
4 and fluorosulfate esters
5 are capable of achieving such alkylations; however, the unavailability of such alkylating agents except for the very simple alkyl groups (
e.g., methyl and ethyl) does not allow generalization. On the other hand, alkyl halides complexed to silver salts form powerful alkylating agents and allow utilization of a wide range of alkyl halides susceptible to S
N2 displacement.
2,6 Although alkyl bromides may be employed, alkyl iodides are preferred. The latter are normally available in excellent yields from sulfonate esters, chlorides, or bromides by reaction with
sodium iodide. Polyhalides may be employed without complications—reaction occurring preferably at a primary rather than secondary center. A nonsilver salt-based method for preparing
3-chloropropyldiphenylsulfonium tetrafluoroborate has also been reported recently.
7While sulfonium ylides do not normally undergo alkylations (except with reactive alkylating agents such as
methyl iodide8), they do undergo intramolecular alkylation (cyclization) rather efficiently. The present procedure describes the synthesis of a particularly interesting reagent,
cyclopropyldiphenylsulfonium tetrafluoroborate.
9 The ylide derived from this salt effects many different synthetic transformations which include facile syntheses of cyclobutanones,
9 γ-butyrolactones,
10 and specifically substituted cyclopentanones
11 from aldehydes and ketones and spiropentanes from α,β-unsaturated carbonyl partners.
9,12 Two reviews of the synthetic applications of the ylide have appeared.
13
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
sodium benzophenone ketyl
terahydrofuran
ethanol (64-17-5)
ether,
diethyl ether (60-29-7)
sodium carbonate (497-19-8)
sodium bromide (7647-15-6)
sodium sulfate (7757-82-6)
sodium thiosulfate (7772-98-7)
nitrogen (7727-37-9)
sulfur (7704-34-9)
acetone (67-64-1)
decolorizing carbon (7782-42-5)
Benzophenone (119-61-9)
sodium (13966-32-0)
1-bromo-3-chloropropane (109-70-6)
Methyl iodide (74-88-4)
sodium iodide (7681-82-5)
sulfonium
Nitromethane (75-52-5)
dichloromethane (75-09-2)
Diphenyl sulfide (139-66-2)
Tetrahydrofuran (109-99-9)
lithium aluminium hydride (16853-85-3)
sodium hydride (7646-69-7)
sodium tetrafluoroborate (13755-29-8)
Cyclopropyldiphenylsulfonium tetrafluoroborate,
Sulfonium, cyclopropyldiphenyl tetrafluoroborate(1-) (33462-81-6)
1-chloro-3-iodopropane (6940-76-7)
silver tetrafluoroborate,
silver fluoroborate (14104-20-2)
3-Chloropropyldiphenylsulfonium tetrafluoroborate (33462-80-5)
diphenylsulfonium
fluoroboric acid (16872-11-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved