Org. Synth. 1973, 53, 59
DOI: 10.15227/orgsyn.053.0059
A GENERAL SYNTHESIS OF 4-ISOXAZOLECARBOXYLIC ESTERS: ETHYL 3-ETHYL-5-METHYL-4-ISOXAZOLECARBOXYLATE
[4-Isoxazolecarboxylic acid, 3-ethyl-5-methyl-, ethyl ester]
Submitted by John E. McMurry
1
Checked by U. P. Hochstrasser and G. Büchi.
1. Procedure
Caution! The following reactions should be performed in an efficient hood to protect the experimentalist from noxious vapors (pyrrolidine, phosphorus oxychloride, and triethylamine).
Benzene has been identified as a carcinogen; OSHA has issued emergency standards on its use. All procedures involving benzene should be carried out in a well-ventilated hood, and glove protection is required.
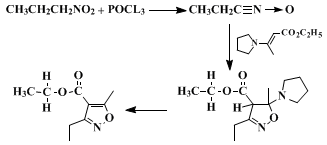
A. Ethyl β-pyrrolidinocrotonate. Ethyl acetoacetate (130 g., 1.00 mole) (Note 1) and pyrrolidine (71 g., 1.0 mole) are dissolved in 400 ml. of benzene and placed in a 1-l., one-necked flask fitted with a Dean-Stark water separator on top of which is a condenser fitted with a nitrogen inlet tube. The reaction mixture is placed under a nitrogen atmosphere (Note 2), then brought to and maintained at a vigorous reflux for 45 minutes, at which time the theoretical amount of water (18 ml.) has been collected. The benzene is removed with a rotary evaporator, yielding 180 g. (98%) of highly pure ethyl β-pyrrolidinocrotonate, which may be used without distillation (Note 3).
B. Ethyl 3-ethyl-5-methyl-4-isoxazolecarboxylate. Ethyl β-pyrrolidinocrotonate (183 g., 1.00 mole), 1-nitropropane (115 g., 116 ml., 1.29 mole), and triethylamine (400 ml.) are dissolved in 1 l. of chloroform and placed in a 5-l., three-necked flask fitted with a 500 ml., pressure-equalizing dropping funnel and a gas-inlet tube. The flask is cooled in an ice bath, and its contents are placed under a nitrogen atmosphere. While the contents of the flask are stirred magnetically, a solution of 170 g. (1.11 mole) of phosphorus oxychloride in 200 ml. of chloroform is added slowly from the dropping funnel. After 3 hours, addition is complete and the ice bath is removed. The reaction mixture is allowed to warm to room temperature and stirring is continued for an additional 15 hours.
The reaction mixture is poured into a 4-l. separatory funnel and washed with 1 l. of cold water. The chloroform layer is washed with 6 N hydrochloric acid until the amine bases are removed and the wash remains acidic (Note 4) The chloroform extracts are washed successively with 5% aqueous sodium hydroxide (Note 5) and saturated brine, dried over anhydrous magnesium sulfate, and filtered. The solvent is removed with a rotary evaporator, and the product is distilled under vacuum, yielding 122–130 g. (68–71%) of ethyl 3-ethyl-5-methyl-4-isoxazolecarboxylate, b.p. 72° (0.5 mm.), nD23 1.4615 (Note 6).
2. Notes
1.
The following reagents were used as supplied:
pyrrolidine and triethylamine, Aldrich Chemical Company, Inc.;
ethyl acetoacetate, Eastman Organic Chemicals (white label);
nitropane, Matheson, Coleman and Bell (practical);
phosphorus oxychloride, Matheson, Coleman and Bell (reagent).
2.
Ethyl β-pyrrolidinocrotonate is typical of most enamines in that it discolors rapidly when exposed to air and therefore must be handled under an inert atmosphere.
3.
Distillation is unnecessary and inadvisable, since discoloration usually occurs and there are product losses.
4.
If this acid wash is not done thoroughly, the
triethylamine hydrochloride remaining will sublime during distillation of the product and coat the still with a fluffy white powder. This impurity can be removed from the distillate, however, by a simple water wash.
5.
This alkaline wash removes traces of
ethyl acetoacetate which might form by hydrolysis of unreacted starting material during the preceding acid wash.
6.
The product had the following spectral data: IR: 1725, 1605, 1300 cm.
−1;
1H NMR (CCl
4); δ 1.3 (m, 6H, 2C
H3), 2.6 (s, 3H, C=CC
H3), 2.7 (q, 2H, C
H2CH
3), 4.2 (q, 2H, OC
H2CH
3).
3. Discussion
This procedure is illustrative of a general method for preparing a wide range of pure 3,5-disubstituted-4-isoxazolecarboxylic esters and (by hydrolysis) their acids,
2 free from positional isomers. A wide range of primary nitro compounds and enamino esters can be used,
2,3 and the esters thus obtained are useful as reagents in the isoxazole annelation reaction.
3,4 The only other general synthesis of these compounds involves chloromethylation and oxidation of a suitable 4-unsubstituted isoxazole,
5 but suffers from two difficulties: low yields and the unavailability of starting isoxazole. Most methods of isoxazole formation yield a mixture of positional isomers,
6 but the present method is quite selective. It has been shown that the reaction of a primary nitro compound with a dehydrating agent such as
phosphorus oxychloride produces an intermediate nitrile oxide
7,8 which then undergoes a 1,3 dipolar cycloaddition to the enamine. This addition is remarkably selective with respect to orientation and no isomer formation is detected.
9 The intermediate isoxazoline then loses
pyrrolidine enroute to the final product.
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
sodium hydroxide (1310-73-2)
chloroform (67-66-3)
nitrogen (7727-37-9)
Phosphorus Oxychloride (21295-50-1)
Ethyl acetoacetate (141-97-9)
Triethylamine hydrochloride (554-68-7)
magnesium sulfate (7487-88-9)
pyrrolidine (123-75-1)
triethylamine (121-44-8)
1-nitropropane (108-03-2)
Ethyl 3-ethyl-5-methyl-4-isoxazolecarboxylate,
4-Isoxazolecarboxylic acid, 3-ethyl-5-methyl-, ethyl ester (53064-41-8)
Ethyl β-pyrrolidinocrotonate (54716-02-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved