Org. Synth. 1973, 53, 116
DOI: 10.15227/orgsyn.053.0116
STEREOSELECTIVE SYNTHESIS OF TRISUBSTITUTED OLEFINS: ETHYL 4-METHYL-(E)-4,8-NONADIENOATE
[4,8-Nonadienoic acid, 4-methyl, ethyl ester, trans-]
Submitted by Ronald I. Trust and Robert E. Ireland
1.
Checked by David G. Melillo and Herbert O. House.
1. Procedure
A. 2-Methyl-1,6-heptadien-3-ol. A dry, three-necked, 1-l., round-bottomed flask fitted with a mechanical stirrer, a reflux condenser with a nitrogen inlet tube, and a 125-ml., pressure-equalizing dropping funnel capped with a rubber septum is charged with 15.7 g. (0.646 g.-atom) of magnesium turnings. The flask is dried by heating with a flame while a stream of dry nitrogen is passed through the reaction vessel from the condenser and allowed to exit from a hypodermic needle inserted in the rubber septum. After drying, the hypodermic needle is removed and the flask is allowed to cool; a static nitrogen atmosphere is maintained in the reaction vessel for the remainder of the reaction. A small crystal of iodine and 450 ml. of anhydrous diethyl ether are added (Note 1). A solution of 49.4 g. (0.546 mole) of 4-chloro-1-butene (Note 2) in 50 ml. of anhydrous ether is then added from the dropping funnel, dropwise and with stirring. Sufficient external heat is applied to the reaction flask to keep the temperature of the reaction mixture at about 30°. After approximately 10–50% of the chloride solution has been added, a spontaneous reaction ensues as evidenced by the disappearance of the yellow iodine color, the appearance of a gray color in the reaction solution, and the commencement of gentle refluxing. The external heat is removed, and the remainder of the chloride solution is added at a rate that maintains gentle refluxing. After the addition is complete, the reaction mixture is refluxed for 30 minutes before a solution of 40.1 g. (0.572 mole) of methacrolein (Note 3) in 50 ml. of anhydrous ether is added, dropwise with stirring and refluxing, over 45 minutes. Since the reaction with methacrolein is exothermic, the application of external heat may not be necessary to maintain refluxing during this addition. During the addition the reaction mixture usually becomes cloudy. When the addition is complete, the reaction mixture is refluxed with stirring for 1.5 hours before it is cooled in an ice water bath and 250 ml. of 5% hydrochloric acid is added slowly and with stirring (Note 4). The organic layer is separated and the aqueous layer is extracted with four 200-ml. portions of ether. The combined organic solutions are washed successively with 200 ml. of saturated aqueous sodium hydrogen carbonate and 200 ml. of saturated aqueous sodium chloride and dried over anhydrous sodium sulfate. The resulting ether solution is concentrated, and the residual liquid is distilled under reduced pressure, yielding 37.2–47.3 g. (54–69%) of 2-methyl-1,6-heptadien-3-ol as a colorless liquid, b.p. 85–88° (33 mm.), nD25 1.4531–1.4535 (Note 5).
B.
Ethyl 4-methyl-(E)-4,8-nonadienoate. A
500-ml., one-necked, round-bottomed flask containing a
magnetic stirring bar is fitted with a
Claisen adapter, two
thermometers, and a receiving flask as illustrated in
Figure 1. To the flask is added
186 g. (1.15 moles) of ethyl orthoacetate (Note 6),
25.2 g. (0.200 mole) of 2-methyl-1,6-heptadien-3-ol, and
0.70 g. (0.0094 mole) of propionic acid (Note 7). The mixture is heated with stirring to keep the temperature above the liquid at 138–142°. Heating is continued until
ethanol no longer distils from the reaction flask (approximately one hour is required). The reaction mixture is allowed to cool to room temperature, and the excess ortho ester and
propionic acid are removed by distillation under reduced pressure (approximately 50–60° at 20 mm.). The colorless to yellow residual liquid is then distilled under reduced pressure (0.25 mm.,
(Note 8)), giving
32.6–34.6 g. (
83–88%) of
ethyl 4-methyl-(E)-4,8-nonadienoate as a colorless liquid, b.p.
54–55° (0.25 mm.),
nD25 1.4504
(Note 9).
Figure 1. Apparatus for the preparation of ethyl 4-methyl-(E)-4,8-nonadienoate.
2. Notes
1.
After drying, exposure of the reaction vessel and its contents to the atmosphere should be minimized. The
iodine crystal should be added by lifting the dropping funnel, then replacing it quickly. The
ether (anhydrous grade from Mallinckrodt Chemical Works) should be distilled from
lithium aluminum hydride immediately before use and transferred to the reaction vessel with a
stainless-steel cannula or a large hypodermic syringe inserted through the rubber septum.
2.
4-Chloro-1-butene is commercially available from Chemical Samples Company. The checkers employed this material without further purification. The submitters used material prepared from
3-buten-1-ol by a modified procedure of Roberts and Mazur.
2 Since material prepared according to the literature is invariably contaminated with
thionyl chloride, which will interfere with formation of the Grignard reagent, the following modification is recommended. A
two-necked, 200-ml., round-bottomed flask is equipped with a magnetic stirring bar, a
60-ml., pressure-equalizing dropping funnel, and a reflux condenser fitted with a
calcium chloride drying tube. The flask is charged with
49.8 g. (0.691 mole) of 3-buten-1-ol and
1.57 ml. of anhydrous pyridine (distilled from
calcium hydride). With stirring and external cooling (ice water bath),
82 g. (49 ml., 0.69 mole) of thionyl chloride (Matheson, Coleman and Bell commercial grade was used without further purification) is added dropwise over 3.5 hours. On completion of the addition, the mixture is heated under reflux for one hour. The external heating is then momentarily discontinued, and the condenser and dropping funnel are replaced by a stopper and short-path distilling head with receiver. Distillation of the mixture gives an opaque, colorless liquid (b.p. 68°). The crude product is washed with two
20-ml. portions of saturated aqueous sodium hydrogen carbonate solution (frothing) and
20 ml. of saturated brine, then dried over
magnesium sulfate and filtered. The filtrate is distilled, giving
43.4 g. (
67–69%) of
4-chloro-1-butene as a colorless liquid, b.p.
68–70°.
3-Buten-1-ol, although commercially available from Aldrich Chemical Company, Inc., can be prepared economically and in large quantities by the addition of
paraformaldehyde to
allylmagnesium bromide [
Org. Synth., Coll. Vol. 4, 748 (1963)] in ether according to procedures outlined for a similar synthesis [
Org. Synth., Coll. Vol. 1, 188 (1944)]. In the present case, the submitters found it convenient to add the
paraformaldehyde (Matheson, Coleman and Bell commercial grade was dried overnight under reduced pressure and in the presence of
phosphorus pentoxide) directly to the
allylmagnesium bromide solution. After a reaction period of 6 hours at reflux, the previously described [
Org. Synth., Coll. Vol. 1, 188 (1944)] isolation procedure gave
3-buten-1-ol in
56% yield.
3.
Technical grade (90%) methacrolein (Aldrich Chemical Company, Inc.) was distilled (b.p.
67–69°) immediately before use.
4.
Since the
methacrolein is used in excess, frothing is no problem as there is no Grignard reagent remaining after the reaction is completed. Addition of
5% hydrochloric acid causes some coagulation of magnesium salts in the aqueous layer, which can be redissolved by addition of more
5% hydrochloric acid.
5.
The product has the following spectral characteristics: IR (CCl
4), 3620 (free OH), 3480 (associated OH), 1645 (C=C), and 910 cm.
−1 (CH=CH
2); UV (95% C
2H
5OH) end absorption 210 mm (ε 208);
1H NMR (CCl
4), δ 1.2–2.3 (m, 4H, 2C
H2), 1.70 (s, 3H, C
H3), 2.93 (broad, 1H, O
H), 4.00 (t,
J = 6 Hz., 1H, OC
H), 4.6–5.2 (m, 4H, vinyl C
H), and 5.5–6.1 (m, 1H, vinyl C
H);
m/e (rel. int.), 111(28), 84(29), 83(21), 71(51), 71(100), 69(23), 67(28), 57(30), 55(51), 43(71), 41(49), and 39(37).
6.
Ethyl orthacetate, available from Aldrich Chemical Company, Inc., was distilled before use. A large forerun was collected, consisting of hydrolysis products of the ortho ester. Material boiling at
135–142° is suitable for use in the reaction. It is convenient to transfer the material to the reaction flask with a stainless-steel cannula to avoid its exposure to atmospheric moisture. A fivefold excess of the ortho ester is needed, since the first step in the reaction is probably the reversible acid-catalyzed exchange of
2-methyl-1,6-heptadien-3-ol with
ethanol.
37.
Practical grade propionic acid (Matheson, Coleman and Bell) was distilled before use (b.p.
141°).
8.
On two occasions, the submitters noticed a sublimable solid crystallizing in the distilling head just before the product began to distill. The distilling head was rinsed with
ether, dried, and replaced, and the distillation was continued. The checkers observed the same phenomenon.
9.
The product has the following spectral properties: IR (CCl
4) 1735 (ester C=O), 1645 (C=C), and 920 cm.
−1 (CH=CH
2); UV (95% C
2H
5OH) end absorption 210 nm (ε 1960);
1H NMR (CDCl
3), δ 1.24 (t,
J = 7 Hz., 3H, OCH
2C
H3), 1.63 (broad, 3H, C=CC
H3), 1.9–2.2 (m, 4H, 2C
H2), 2.37 (broad, 4H, 2C
H2), 4.14 (q,
J = 7 Hz., 2H, OC
H2CH
3), 4.8–5.4 (m, 3H, vinyl C
H), 5.5–6.2 (m, 1H, vinyl C
H);
m/e (rel. int.), 196 (M
+, 4), 155(67), 151(30), 113(34), 109(100), 108(33), 85(47), 81(80), 67(74), 55(41), 53(31), 43(32), and 41(30). In C
6D
6 the allylic CH
3 signal of the major component present, the
trans-isomer, is found at δ 1.50 and is accompanied by a minor peak at δ 1.61 attributable
4 to 3–4% of the
cis-olefin in the product.
3. Discussion
The use of the Claisen rearrangement and several other methods for the stereoselective synthesis of trisubstituted olefins has been reviewed.
4 In allyl vinyl ethers of type A, the stereochemistry of the rearrangement is determined largely by the steric requirements of R
1, which can be either axial or equatorial in the transition state.
5 When R
3 = H, the
trans/cis ratio is approximately equal to the equatorial/axial equilibrium ratio of R
1-cyclohexane at the reaction temperature. When R
3 is larger than
hydrogen, the steric effect is even greater, due to a potential 1,3-interaction which would develop in the transition state if R
1 were axial. No significant effect of R
2 on the
trans/cis ratio has been observed.
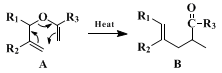
The use of
ethyl orthoacetate in the formation of vinyl ethers where R
3 = OC
2H
5 has been described.
3,6 The method described herein appears to be quite general, in that a variety of esters of type B (R
3 = OC
2H
5; R
2 = CH
3) may be prepared by merely varying the Grignard reagent used in preparing the starting
allyl alcohol. The only limitation is the use of alcohols that are unsymmetrically bis-allylic, from which mixtures of structural isomers may be obtained.
Stereoselectivity in the synthesis of trisubstituted olefins is necessary for the study of biosynthetic routes to polyisoprenoids, the nonenzymatic cyclization of polyolefinic substrates, and the study of insect hormones.
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
Ethyl orthacetate
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
ether,
diethyl ether (60-29-7)
hydrogen (1333-74-0)
thionyl chloride (7719-09-7)
sodium hydrogen carbonate (144-55-8)
magnesium turnings (7439-95-4)
sodium chloride (7647-14-5)
propionic acid (79-09-4)
Allyl alcohol (107-18-6)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
iodine (7553-56-2)
pyridine (110-86-1)
magnesium sulfate (7487-88-9)
lithium aluminum hydride (16853-85-3)
Allylmagnesium bromide (1730-25-2)
methacrolein (78-85-3)
calcium hydride (7789-78-8)
3-buten-1-ol (627-27-0)
4-chloro-1-butene (927-73-1)
2-Methyl-1,6-heptadien-3-ol (53268-46-5)
ethyl orthoacetate
phosphorus pentoxide (1314-56-3)
Ethyl 4-methyl-(E)-4,8-nonadienoate,
4,8-Nonadienoic acid, 4-methyl, ethyl ester, trans- (53359-96-9)
paraformaldehyde (30525-89-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved