Org. Synth. 1970, 50, 97
DOI: 10.15227/orgsyn.050.0097
1-PHENYL-1,4-PENTADIYNE AND 1-PHENYL-1,3-PENTADIYNE
[Benzene, 1,4-pentadiynyl- and Benzene, 1,3-pentadiynyl-]
Submitted by H. Taniguchi, I. M. Mathai, and Sidney I. Miller
1.
Checked by Marion F. Habibi and Richard E. Benson.
1. Procedure
A. Phenylethynylmagnesium bromide. A 1-l., four-necked flask fitted with a sealed mechanical stirrer, a reflux condenser carrying calcium chloride and soda lime tubes, a nitrogen gas inlet, and a dropping funnel is charged with 19 g. (0.81 g.-atom) of magnesium. The flask is flushed with prepurified nitrogen, the stirrer is started, and 109 g. of ethyl bromide (1.00 mole) in 350 ml. of anhydrous tetrahydrofuran (Note 1) is added. After the magnesium has dissolved (Note 2), 102 g. (1.00 mole) of phenylacetylene (Note 3) in 150 ml. of tetrahydrofuran is added over a period of ca. 30 minutes at a rate that maintains a gentle reflux (Note 4). The reaction mixture is then heated at reflux for ca. 1.5 hours (Note 5).
B. 1-Phenyl-1,4-pentadiyne. Anhydrous copper(I) chloride (2 g.) is added to the flask, and heating under reflux is continued for 20 minutes. At this point 96 g. (0.81 mole) of propargyl bromide in 120 ml. of tetrahydrofuran (Note 1) and (Note 6) is added over 30–40 minutes at a rate that maintains a gentle reflux. The mixture, containing a yellow solid, is heated another 30–40 minutes, allowed to cool to ambient temperature, and poured slowly into 2 l. of ice-water slush containing 50 ml. of concentrated sulfuric acid. The whole mixture, which should be acidic at this point, is stirred thoroughly and extracted five times with 200-ml. portions of diethyl ether (Note 7). The ether extracts are combined and washed with 100-ml. portions of water until the water layer is no longer acidic to litmus paper (3–4 washings are usually required). The ether layer is dried over magnesium sulfate overnight, separated from the desiccant, and concentrated by distillation.
The product is distilled under nitrogen using a 25-cm. Vigreux column (Note 8) and (Note 9). After removal of unreacted propargyl bromide and phenylacetylene, 51–64 g. (45–57%) of 1-phenyl-1,4-pentadiyne is collected as the main fraction, b.p. 64–66° (0.45 mm.), nD25 1.5713 (Note 9) and (Note 10). The colorless product is best stored under nitrogen at ca. −78°. The 1H NMR spectrum (60 mHz., neat, external tetramethylsilane reference) shows peaks at δ 1.76 (t, 1H), 2.92 (d, 2H), and 6.87 (m, 5H) (Note 11).
C. 1-Phenyl-1,3-pentadiyne. A flask containing a magnetic stirrer and a solution of 2 g. of sodium hydroxide in 50 ml. of ethanol is flushed with nitrogen, and 10 g. (0.071 mole) of 1-phenyl-1,4-pentadiyne is added. The flask is stoppered, and the contents are stirred for ca. 2 hours. The brown solution is then poured into 200 ml. of water, and the mixture extracted four times with 50-ml. portions of ether. The extracts are combined and washed with two 50-ml. portions of water. The ether layer is dried over magnesium sulfate, filtered, and concentrated by distillation. The product is distilled as described in Part B, yielding 5.4–7.5 g. (54–75%) of 1-phenyl-1,3-pentadiyne as a colorless oil, b.p. 62° (0.15 mm.), nD25 1.6324. The 1H NMR spectrum (60 mHz., neat, external tetramethylsilane reference) shows peaks at δ 1.47 (s, 3H) and 6.87 (m, 5H) (Note 11).
2. Notes
1.
Reagent grade tetrahydrofuran available from Fisher Scientific Company was used by the checkers. For warning regarding the purification of
tetrahydrofuran see
Org. Synth., Coll. Vol. 5, 976 (1973).
2.
The flask may be warmed to hasten the dissolution of
magnesium.
3.
Phenylacetylene was distilled under
nitrogen, b.p.
91–92° (17.5 mm.).
4.
An
ice-water bath should be kept at hand to cool the flask should the refluxing become too vigorous.
5.
If the reaction is slow, the solution is kept at reflux overnight under an atmosphere of
nitrogen. More
tetrahydrofuran (50 ml.) may be added if stirring becomes difficult. It is desirable that procedure B follow A as soon as possible.
6.
Propargyl bromide was redistilled, b.p.
82–83°.
7.
The diyne discolors at room temperature and on exposure to air. Therefore, it is desirable to proceed without delay in the workup steps.
8.
The checkers found it advantageous to do a crude preliminary distillation using a vapor-bath still. The resulting distillate can be redistilled using a
spinning band column. This procedure appeared to avoid an exothermic reaction that occurred when the bath temperature rose above 110°
(Note 9).
9.
After the 1,4-diyne has distilled, overheating the pot contents is to be avoided since an exothermic reaction can occur.
10.
The checkers observed yields of
44–50%, conducting the reaction on a scale one-half that described here.
11.
Where they differ from those reported previously,
2 the values of the physical properties are those obtained by the checkers.
3. Discussion
The synthetic route described here has been used for various "skipped" diynes and related 1,3-diynes, among which are precursors or analogs of naturally occurring polyynes.
2 The
copper(I) chloride-promoted coupling reaction in
tetrahydrofuran provides the best and often the sole route to 1,4-diynes.
2 From these it is simple to proceed to the 1,3-diyne and, possibly, the isomeric allene.
2,3 Because all of these compounds appear to be sensitive to heat and
oxygen the relatively mild reaction conditions are noteworthy.
1-Phenyl-1,4-pentadiyne has been prepared by coupling in
tetrahydrofuran without a
copper chloride catalyst in
22% yield,
4 and in
ether with a
copper chloride catalyst in
28% yield.
5 In general, the coupling of propargyl halides with various metallic acetylides,
e.g., sodium, silver, gives 1,4-pentadiynes in low yields at best.
2 For example, 1,4-pentadiyne was prepared from
propargyl bromide and
acetylenemagnesium bromide in
tetrahydrofuran in
20% yield.
6 Strongly basic reactants such as alkali acetylides or basic conditions for workup and purification,
e.g., column chromatography over alumina, promote the isomerization of the 1,4-diyne.
2,3 The evolution of the present method, which emphasizes the use of
tetrahydrofuran as solvent, a copper(I) salt, a short coupling time, and neutral reaction and workup conditions, has been described in detail.
2
1-Phenyl-1,3-pentadiyne has been prepared by the dehydrobromination of the corresponding butadiene tetrabromide.
7 Other unsymmetrical 1,3-diynes have been prepared, as described in scheme 1.
8 It is, of course, typical of crossed-coupling reactions that some should proceed as desired in (2),
2 and that others should go astray, as in (3).
2 The conversion of 1,4- to 1,3-diynes by strong base in
ethanol or
methanol at reflux has been described.
4,5 As judged by the isomerization rates in
sodium ethoxide–ethanol, heating appears to be unnecessary. Typical rate constants are given in (4).
3
The present route to conjugated diynes is the method of choice
if the corresponding skipped diyne or allene is available.
2,3 With naturally occurring polyynes this is often the case. Depending on the 1,4-diyne, the base-catalyzed isomerization may be successfully stopped (by acidification), yielding the intermediate allenylacetylene. Since the isolation of the allene may or may not be desired, it seems prudent to monitor the progress of isomerization of new 1,4-diynes,
e.g., by
1H NMR, and establish the approximate lifetimes of transient species. In this way allenes as well as conjugated diynes may be obtained.
3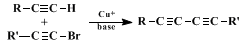
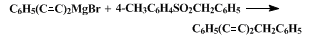
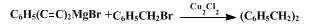
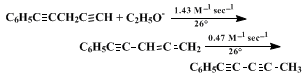
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
ethanol (64-17-5)
sulfuric acid (7664-93-9)
methanol (67-56-1)
ether,
diethyl ether (60-29-7)
sodium hydroxide (1310-73-2)
magnesium (7439-95-4)
Ethyl bromide (74-96-4)
oxygen (7782-44-7)
nitrogen (7727-37-9)
sodium ethoxide (141-52-6)
copper(I) chloride (7758-89-6)
copper chloride (7758-89-6)
Phenylacetylene (536-74-3)
magnesium sulfate (7487-88-9)
Tetrahydrofuran (109-99-9)
phenylethynylmagnesium bromide
propargyl bromide (106-96-7)
1-Phenyl-1,4-pentadiyne,
Benzene, 1,4-pentadiynyl- (6088-96-6)
1-Phenyl-1,3-pentadiyne,
Benzene, 1,3-pentadiynyl- (4009-22-7)
acetylenemagnesium bromide
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved