Org. Synth. 1988, 66, 8
DOI: 10.15227/orgsyn.066.0008
A GENERAL [3+2] ANNULATION: cis-4-exo-ISOPROPENYL-1,9-DIMETHYL-8-(TRIMETHYLSILYL)BICYCLO[4.3.0]NON-8-EN-2-ONE
[4H-Inden-4-one, 1,3a,5,6,7,7a-hexahydro-3,3a-dimethyl-6-(1-methylethenyl)-2-(trimethylsilyl)-, (3aα,6α,7aα)-]
Submitted by Rick L. Danheiser, David M. Fink, and Yeun-Min Tsai
1.
Checked by Marianne Marsi and Bruce E. Smart.
1. Procedure
A 500-mL, three-necked, round-bottomed flask is equipped with a 25-mL pressure-equalizing dropping funnel, a mechanical stirrer, and a Claisen adapter fitted with a nitrogen inlet adapter and a low-temperature thermometer (Note 1). The flask is charged with 115 g (0.077 mol) of (R)-(−)-carvone (Note 2), 10.8 g (0.079 mol) of 1-methyl-1-(trimethylsilyl)allene (Note 3), and 180 mL of dry dichloromethane (Note 4), and then cooled below −75°C with a dry ice–acetone bath while a solution of 17.4 g (0.092 mol) of titanium tetrachloride (Note 5) in 10 mL of dichloromethane is added dropwise over 1 hr. After 30 min, the cold bath is removed, and the reaction mixture, which appears as a red suspension, is allowed to warm to 0°C over approximately 30 min. The resulting dark red solution is poured slowly into a 2-L Erlenmeyer flask containing a magnetically stirred mixture of 400 mL of diethyl ether and 400 mL of water (Note 6). The aqueous phase is separated and extracted with two 200-mL portions of ether. The combined organic phases are washed with 250 mL of water and 250 mL of saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and concentrated at reduced pressure using a rotary evaporator. The residual yellow liquid is distilled through a 15-cm Vigreux column at reduced pressure to afford 17.5 g (82%) of the bicyclonoenone 1 as a very-pale-yellow liquid, bp 98–101°C (0.03 mm), [α]D20 −157.8 ± 0.8 (1.57, CH2Cl2) (Note 7) and (Note 8).
2. Notes
1.
The apparatus is flame-dried under vacuum and then maintained under an atmosphere of
nitrogen during the course of the reaction.
2.
(R)-(−)-Carvone was purchased from Aldrich Chemical Company, Inc. and distilled before use.
3.
1-Methyl-1-(trimethylsilyl)allene (90% purity, contaminated with 10% 1-trimethylsilyl-1-butyne) was prepared by the method of Danheiser, R. L.; Tasai, Y. M.; Fink, D. M.
Org. Synth., Coll. Vol. VIII, 1993, 471.
4.
Dichloromethane was distilled from
calcium hydride immediately before use.
5.
Titanium tetrachloride (99.9%) was obtained from the Aldrich Chemical Company, Inc. and distilled before use. Lower yields (70–77%) resulted if the
titanium tetrachloride was not distilled. Unreacted
carvone is recovered if a small excess of
titanium tetrachloride is not used.
6.
The two-phase mixture is vigorously stirred using a
7-cm Teflon-coated magnetic stirring bar.
7.
The submitters report obtaining
18.8 g (
88%) of product, bp
93–96°C (0.03 mm). The purity of the product was determined to be >99% by gas chromatographic analysis (10% OV-101 on 100-120 mesh Chromosorb W, 6 ft × 1/8 in., program: 200°C for 2 min and then 200–300°C at 32°C/min).
8.
The product exhibits the following spectral properties: IR (neat) cm
−1: 3080, 2950, 2920, 1700, 1640, 1610, 1440, 1375, 1315, 1245, 1200, 830, 755, 680;
1H NMR (250 MHz, CDCl
3) δ: 0.08 (s, 9 H), 1.10 (s, 3 H), 1.65 (t, 3 H,
J = 2.2), 1.68 (m, 3 H), 1.65–1.72 (m, 2 H), 2.10 (d of m, 1 H,
J = 12.4), 2.15–2.25 (m, 1 H), 2.27–2.31 (m, 2 H), 2.45–2.57 (m, 2 H), 4.63 (m, 1 H), 4.72 (m, 1 H);
13C NMR (62.8 MHz, CDCl
3) δ: −0.7, 14.4, 21.1, 21.3, 32.4, 39.8, 42.0, 43.9, 46.1, 64.8, 110.4, 136.1, 147.7, 151.5, 215.6.
3. Discussion
The procedure described here serves to illustrate a general [3 + 2] annulation method
2 3 for the synthesis of cyclopentane derivatives. A unique feature of this one-step annulation is its capacity to generate regiospecifically five-membered rings substituted at each position, and functionally equipped for further synthetic elaboration. As formulated in the following equation, the reaction proceeds with remarkably high stereoselectivity via the effective suprafacial addition of the three-carbon allene component to an electron-deficient olefin ("allenophile").
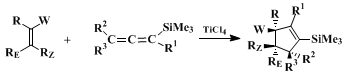
Some representative examples of the [3 + 2] annulation are listed in Table I. Both cyclic and acyclic allenophiles participate in the reaction. α-Alkylidene ketones undergo annulation to provide access to spiro-fused systems, and acetylenic allenophiles react to form cyclopentadiene derivatives. The reactions of
(E)- and (Z)-3-methyl-3-penten-2-one illustrate the stereochemical course of the annulation, which proceeds with a strong preference for the suprafacial addition of the
allene to the two-carbon allenophile. The high stereoselectivity displayed by the reaction permits the stereocontrolled synthesis of a variety of mono- and polycyclic systems.
TABLE I
[3 + 2] ANNULATIONS EMPLOYING ALLENYLSILANES
|
|
Allenophile
|
Allene
|
Annulation Product
|
Yield (%)
|
|
|
|
|
|
71-75
|
|
|
|
|
86
|
|
|
|
|
49
|
|
|
|
|
91
|
|
|
|
|
90
|
|
|
|
|
71
|
|
|
|
|
68
|
|
|
|
|
53
|
|
|
|
|
63
|
|
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(R)-(−)-carvone
(E)- and (Z)-3-methyl-3-penten-2-one
ether,
diethyl ether (60-29-7)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
Allene (463-49-0)
titanium tetrachloride (7550-45-0)
calcium hydride (7789-78-8)
carvone
1-Methyl-1-(trimethylsilyl)allene (74542-82-8)
1-trimethylsilyl-1-butyne
cis-4-exo-Isopropenyl-1,9-dimethyl-8-(trimethylsilyl)bicyclo[4.3.0]non-8-en-2-one (77494-23-6)
4H-Inden-4-one, 1,3a,5,6,7,7a-hexahydro-3,3a-dimethyl-6-(1-methylethenyl)-2-(trimethylsilyl)-, (3aα,6α,7aα)-
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved