Org. Synth. 1987, 65, 183
DOI: 10.15227/orgsyn.065.0183
ASYMMETRIC SYNTHESES USING THE SAMP-/RAMP-HYDRAZONE METHOD: (S)-(+)-4-METHYL-3-HEPTANONE
[3-Heptanone, 4-methyl, (S)-]
Submitted by Dieter Enders, Helmut Kipphardt, and Peter Fey
1.
Checked by Benjamin Guzmán, Stan S. Hall, and Gabriel Saucy.
1. Procedure
Caution! Ozone is extremely toxic and can react explosively with certain oxidizable substances. Ozone also reacts with some compounds to form explosive and shock-sensitive products. Ozone should only be handled by individuals trained in its proper and safe use and all operations should be carried out in a well-ventilated fume hood behind a protective safety shield. [Note added September 2009].
A. 3-Pentanone SAMP hydrazone [(S)-2]. A 50-mL, one-necked, pear-snapped, flask equipped with a 10-cm Liebig condenser, a gas inlet tube, and a magnetic stirring bar is charged with 3.9 g (3.0 mmol) of SAMP (Note 1) and 3.79 mL (36 mmol) of 3-pentanone (Note 2) and the mixture is warmed at 60°C under argon overnight (Note 3). The crude product is diluted with 200 mL of ether in a 250-mL separatory funnel and washed with 30 mL of water. The organic layer is separated, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Purification by short-path distillation yields 5.18 g (87%) of a colorless oil, bp 70–75°C at 0.5 mm, [α]D20 +297° (benzene, c = 1). The SAMP-hydrazone (S)-2 should be stored in a refrigerator under argon (Note 4).
B. (S)-(+)-4-Methyl-3-heptanone SAMP hydrazone [(ZSS)-3]. A flame-dried, one-necked 250-mL flask with side arm, rubber septum, and magnetic stirring bar is flushed with argon (Note 5). The flask is then cooled to 0°C and 110 mL of dry ether (Note 6) and 2.97 mL (21 mmol) of dry diisopropylamine (Note 7) are added, followed by dropwise addition of 21 mmol of butyllithium (13.1 mL of a 1.6 N solution in hexane (Note 8)). Stirring is continued for 10 min and a solution of 3.96 g (20 mmol) of SAMP-hydrazone (S)-2 in 10 mL of ether is added to the stirred mixture over a 5-min period at 0°C. An additional 2 mL of ether is used to transfer all of the hydrazone (S)-2 into the reaction flask. Stirring is continued for 4 hr at 0°C, while the lithiated hydrazone precipitates. The mixture is cooled to −110°C (pentane/liquid nitrogen bath) and kept for 15 min at this temperature. Then 2.15 mL (22 mmol) of propyl iodide (Note 9) is added dropwise, and the mixture is allowed to reach room temperature overnight. The contents of the flask are poured into a mixture of 300 mL of ether plus 50 mL of water in a 500-mL separatory funnel, the layers are separated, and the aqueous layer is extracted twice with 25 mL of ether. The combined organic layers are washed with 10 mL of water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to yield 4.3 g (90%) of crude (ZSS)-3 (Note 10).
C. (S)-(+)-4-Methyl-3-heptanone [(S)-4]. A 100-mL Schlenk tube, fitted with a gas inlet and Teflon stopcocks, is charged with 4.3 g (18 mmol) of crude (ZSS)-3 dissolved in 50 mL of dichloromethane (Note 11), and cooled to −78°C (acetone/dry ice bath) under nitrogen. Dry ozone (Note 12) is passed through the yellow solution until a green-blue color appears (ca. 4 hr). The mixture is then allowed to come to room temperature while a stream of nitrogen is bubbled through the solution to give the yellow nitrosamine (S)-5 (Note 13) and the title ketone (S)-4. The solvent is removed by distillation at 760 mm (60°C bath temperature) and the residue is transferred into a microdistillation apparatus (10-mL flask, 5-cm Vigreux column, spider, collector device, (Note 14)). After a small forerun (3–4 pale-yellow drops), a colorless liquid distills to afford 1.6–1.7 g (56–58% overall) of ketone (S)-4; bp 63–67°C at 40 mm (110–115°C bath temperature), GLC analysis 98.2%, [α]D20 + 21.4° to + 21.7° (hexane, c = 2.2), [α]D20 + 17.88° (neat) (Note 15). To recycle the chiral auxiliary SAMP, see (Note 16).
2. Notes
1.
(S)-1-Amino-2-methoxymethylpyrrolidine (SAMP) and RAMP are commercially available from Merck-Schuchardt (Frankfurter Strasse 250, 6100 Darmstadt or Eduard-Buchner-Strasse 14-20, 8011 Hohenbrunn, Germany) and Aldrich Chemical Company, Inc., Milwaukee, Wisconsin. The submitters prepared
SAMP from
(S)-proline as described in
Org. Synth., Coll. Vol. VIII 1993, 26; the checkers used SAMP from Merck-Schuchardt.
2.
Redistilled prior to use,
3-pentanone was obtained from Merck-Schuchardt (submitters) and Aldrich Chemical Company, Inc. (checkers).
3.
The reaction was monitored by TLC. The TLC plates (SiO
2, F
254, 0.25 mm), commercially available from Merck, Darmstadt, Germany, were eluted with
ether and developed by dipping into a
10% ethanolic solution of phosphomolybdic acid (Merck) and then heating.
24.
Partial
1H NMR spectrum (CDCl
3, 200 MHz) δ: 1.06 (t, 3 H,
J = 7.7, CH
3CH
2), 1.08 (t, 3 H,
J = 7.5, CH
3CH
2), 3.34 (s, 3 H, CH
3O); IR (film) cm
−1: 1645.
5.
This is done by alternately evacuating and filling the flask with
argon 3 times. During the reaction a pressure of about 50 mm above atmospheric pressure is maintained using a
mercury bubbler. All reagents are added via a glass syringe under rigorously anhydrous conditions. For a more detailed description of the metalation technique, see
3.
6.
The submitters used
ether that had been freshly distilled from
sodium and
benzophenone under an
argon atmosphere. The checkers used anhydrous
ether directly from freshly opened
500-g containers from Fisher Scientific Company, Springfield, NJ. In addition, the checkers charged the reaction flask with
ether using a dry graduated cylinder, flushed the system with
argon, and then sealed and cooled the vessel to 0°C before the sequential addition of
diisopropylamine and
butyllithium.
7.
Diisopropylamine from BASF AG, Ludwigshafen, Germany (submitters) and Aldrich Chemical Company, Inc. (checkers) was distilled from
calcium hydride, and then stored under
argon and over
calcium hydride until use.
8.
Butyllithium was purchased from Metallgesellschaft, Frankfurt, Germany, and titrated for active alkyllithium using
diphenylacetic acid as an indicator.
4 The checkers used fresh
butyllithium, 1.55 M in hexane under argon, from Aldrich Chemical Company, Inc. and omitted the titration.
9.
Propyl iodide was obtained from Merck-Schuchardt, distilled over
potassium carbonate, and stored over
copper wire under
argon.
Caution: Propyl iodide is a cancer suspect agent. The checker's source was Aldrich Chemical Company, Inc.
10.
Partial
1H NMR spectrum (CDCl
3, 200 MHz) δ: 0.88 (t, 3 H,
J = 6.9, CH
3CH
2), 1.02 (d, 3 H,
J = 7.1, CH
3CH), 1.10 (t, 3 H,
J = 7.5, CH
3CH
2), 3.33 (s, 3 H, CH
3O); IR (film) cm
−1: 1630. An
1H-NMR experiment using the chiral shift reagent [Eu(hfc)
3, Aldrich] with crude
3 shows that only the (
ZSS) isomer is present (sharp methoxy singlet). During the measurement a slow isomerization to the thermodynamically more stable (
ESS) isomer takes place, but within the limit of detection of a 100-MHz spectrometer no (
SR) diastereomer can be seen [diastereomeric excess (de) > 97%].
5 611.
Dichloromethane was distilled over
potassium carbonate prior to use.
12.
Caution! Organic ozonides are highly explosive. The reaction should be carried out in a well-ventilated hood with a shatter-proof shield. Do not grease the ground-glass joints! The submitters used a Fischer Model OZ II ozonizer from Fischer, Bad Godesberg, Germany. For detailed descriptions of a laboratory ozonizer see
Org. Synth., Coll. Vol. III, 1955, 673. The checkers used a Welsbach Model T-408 Laboratory Ozonizer, Welsbach Corp., Philadelphia, PA. The power setting was 100 V (AC) and the
oxygen pressure setting was 8 psi 0.55 kg/cm
2) to produce 2–3%
ozone at a gas flow rate (rotameter) of 2 L/min. The
ozone production rate was measured by passing a measured amount of ozonized gas through a
2% potassium iodide solution (neutral), acidifying with 1
M sulfuric acid, and then titrating the liberated
iodine with 0.1
N sodium thiosulfate. Using these conditions, the ozonolysis required at least 4 hr, rather than the 30 min suggested by the submitters.
13.
Caution! The nitrosamine (S)-5 may be carcinogenic. All operations with (S)-5 should be performed in a well-ventilated hood, and the operator should wear disposable gloves. In order to destroy any
nitrosamine traces, the glassware contaminated with (
S)-
5 should be immersed in a
bath of HBr/acetic acid.
14.
To prevent any racemization during distillation, the apparatus is shaken with 1 mL of
chlorotrimethylsilane (freshly distilled from
calcium hydride), which is removed under reduced pressure.
Caution: Glassware, cleaned under alkaline conditions, will lead to spontaneous racemization! The spider (three to four arms for liquid collection) should be cooled with an
ice bath. To prevent bumping, the checkers performed the distillation with a
Bunsen burner rather than with a bath.
15.
The product has an optical purity of 97–98% by comparison with the reported optical rotation of
[α]D25 + 22.1 ± 0.4° (
hexane,
c = 1.0) of the naturally occurring pheromone
7 and an ee of ≥97% by comparison with the de of ≥97% of (
ZSS)-
3 (Note 10);
1H NMR (CDCl
3, 200 MHz) δ: 0.90 (t, 3 H,
J = 6.7, CH
3CH
2), 1.06 (d, 3 H,
J = 6.9, CH
3CH) superimposed on 1.04 (t, 3 H,
J = 7.2, CH
3CH
2), 2.45 (q, 2 H,
J = 7.3, CH
2CH
3); IR (film) cm
−1: 1710.
16.
The nitrosamine (
S)-
5 (
1.94 g,
67%) is obtained from the residue of the ketone distillation (bp
79–80°C/0.1 mm). Reduction with
lithium aluminum hydride in
tetrahydrofuran yields
1.47 g (
49% overall) of SAMP
8 9 with an
[α]D20 −75.46° (neat).
3. Discussion
The title ketone (
S)-
4, which is 400 times more active than its enantiomer,
7,10 is the principal alarm pheromone of the leaf-cutting ant
Atta texana. It has also been identified as an alarm pheromone in three other ant genera of the subfamily
Myrmicinae,
7,11 as a component of the defensive secretion of the "daddy longlegs"
Leiobunum vittatum (Opiliones),
12,13 and is produced by the elm bark beetles
Scolytus scolytus (F.) and
S. multistriatus.14The ketone (
S)-
4 and/or its enantiomer (
R)-
4 have been prepared via resolution of an intermediate,
10 starting from
(R)-citronellic acid,
15 by stoichiometric asymmetric synthesis
4,5,6,7,8,9,10,11,12,13,14,15,16,17,18 19 (76–88% ee), and by a microbiological method.
20The three-step procedure described here, using inexpensive, commercially available starting materials and the chiral auxiliary SAMP, illustrates the synthetic utility of the "SAMP-/RAMP-hydrazone method."
21 22 23 24 25 It is remarkable that the classical electrophilic substitution of a conformationally flexible, acyclic ketone
1 → (
S)-
4 occurs with virtually complete asymmetric induction. This demonstrates complete stereochemical control of the three critical operations: metalation, alkylation, and cleavage. Because deprotonated SAMP-/RAMP-hydrazones react with nearly the entire palette of electrophiles, this new methodology, a chiral version of the now widely used dimethylhydrazone (DMH) method,
3 opens an elegant and economical entry to a great variety of important classes of compounds with good overall chemical yields and excellent diastereo- and enantioselectivities. The following stereoselective reactions may be mentioned: α-alkylations or aldehydes
8,26 and ketones;
5,6,8,13 diastereo- and enantioselective aldol reactions;
9,27,28 diastereo- and enantioselective Michael additions to form β,γ-substituted δ-keto esters,
29,30 δ-lactones,
31 and various heterocycles, such as dihydropyridines, octahydroquinolinediones and hexahydroquinolinones;
32 33 α-alkylations of β-keto esters;
21,22,23,24,25 and, finally, asymmetric syntheses of α- and/or β-substituted primary amines
23,24 via alkylation–reductive amination of aldehydes and ketones
34 or nucleophilic addition to aldehyde-SAMP-/RAMP-hydrazones, followed by N-N bond cleavage.
35 This broad applicability is summarized in
Figure 1, and typical examples are listed in Table I.
Figure 1. Optically active carbonyl compounds and amines.
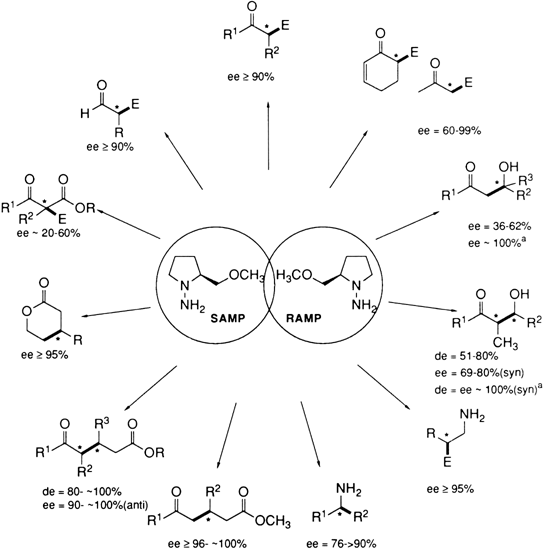
TABLE I
OPTICALLY ACTIVE CARBONYL COMPOUNDS AND AMINES PREPARED BY ASYMMETRIC SYNTHESIS USING THE SAMP/RAMP HYDRAZONE METHOD
|
|
Carbonyl Compound Amine
|
Electrophile
|
Cleavagea
|
Yieldb [%]
|
ee[%] (Config.)
|
Lit.
|
|
|
|
C2H5I
|
B
|
71 |
95 (S)
|
8,21
|
|
|
CH3I
|
A
|
65 |
95 (R)
|
8,21
|
|
|
C6H13I
|
A
|
52 |
≥95 (S)
|
8,21
|
|
|
CH3I
|
A
|
61 |
95 (R)
|
8,21
|
|
|
(CH3)2SO4
|
B
|
65 |
95 (R)
|
21,36
|
|
|
(CH3)2SO4
|
B
|
51 |
95(S)c
|
21,36
|
|
|
(CH3)2SO4
|
A
|
66 |
86 (R)
|
8
|
|
CH3I
|
A
|
74 |
45 (R)
|
8
|
|
|
(CH3)2SO4
|
A
|
70 |
~99 (R)
|
8
|
|
CH3I
|
A
|
70 |
67 (R)
|
8
|
|
|
CH3I
|
B
|
59 |
94 (R)
|
8
|
|
|
CH3I
|
C
|
43 |
93 (R)
|
21,23
|
|
|
H2C=CH(CH2)2Br
|
B
|
26 |
≥89 (R)
|
21,37
|
|
|
|
B
|
61 |
≥97 (S)
|
13
|
|
|
|
B
|
62 |
≥97 (R)c
|
13
|
|
|
|
B
|
46 |
99 (S)
|
38
|
|
|
|
A
|
53 |
>95 (S)
|
21,23
|
|
|
|
A
|
80 |
≥95 (S)
|
21,23
|
|
|
C2H5I
|
B
|
44 |
≥97 (S)
|
6
|
|
|
c-C6H11CHO
|
D
|
32 |
62 (-) ~100 (-)e
|
27
|
|
|
C5H11CHO
|
E
|
75 |
39 (S)c
|
9
|
|
|
C6H5CHO
|
A
|
86 |
72 (SS) [de=67% (SS)]
|
28
|
|
A
|
35
|
de=ee= ~100 (SS)f
|
|
|
|
C6H5CHO
|
A
|
81 |
74 (SS) [de=66% (SS)]
|
28
|
|
A
|
37 |
de=ee= ~100 (SS)f
|
|
|
|
|
A
|
62 |
≥99 (R)
|
29
|
|
|
|
A
|
45 |
≥96 (R)
|
29
|
|
|
|
A
|
46 |
≥95 (R)
|
31
|
|
|
|
A
|
39 |
≥98 (SS) [de ~ 100% (SS)]
|
30
|
|
|
|
A
|
35g
|
≥95 (R)
|
31
|
|
|
|
A
|
30g
|
≥95 (R)
|
31
|
|
|
CH3I
|
A
|
65
|
60 (-)
|
21,23
|
|
|
C2H5I
|
A
|
52
|
27(+)
|
21,23
|
|
|
CH3I
|
h
|
56
|
>90 (R)
|
34
|
|
|
C6H13Br
|
h
|
63
|
>95 (S)
|
34
|
|
|
|
i
|
60
|
90 (S)
|
23,24
|
|
|
|
j
|
47
|
88 (R)
|
23,24
|
|
|
|
k
|
56
|
85 (R)
|
35
|
|
|
|
l
|
41
|
87 (R)
|
35
|
|
a(A) Oxidative cleavage by ozonolysis (O3, CH2Cl2, −78°C). (B) Acidic hydrolysis [(i) excess MeI, 60°C; (ii) 5 N HCl/pentane]. (C) Acidic hydrolysis (12 N HCl/ether). (D) Oxidative cleavage (1O2, Me2S, hydrolysis). (E) Oxidative cleavage (30% H2O2, MeOH, pH 7 buffer).
|
|
|
cRAMP was used as chiral auxiliary.
|
dDiastereomeric excess of corresponding SAMP-hydrazone.
|
eAfter two recrystallizations of the ketol.
|
fAfter separation and cleavage of the corresponding crystalline SAMP-hydrazone.
|
gOverall yield, including reduction of the intermediate β-substituted aldehyde esters and lactonization.
|
hThe primary amines are obtained by catechol–borane reduction of the SAMP-hydrazones, followed by N-N bond cleavage with Raney nickel.
|
iObtained by LiAlH4 reduction of 3,3-dimethyl-2-butanone-SAMP-hydrazone, followed by N-N bond cleavage.
|
jObtained by catechol–borane reduction of 3,3-dimethyl-2-butanone-SAMP-hydrazone, followed by N-N bond cleavage.
|
kObtained by addition of butyllithium to benzaldehyde-SAMP-hydrazone, followed by N-N bond cleavage.
|
lObtained by addition of methyllithium to 2,2-dimethylpropanal-SAMP-hydrazone, followed by N-N bond cleavage.
|
In S
E2' front-type electrophilic substitutions via SAMP-/RAMP-hydrazones the less substituted α-carbon atom is regioselectively deprotonated. Under the standard reaction conditions (
lithium diisopropylamide, 0°C, ether or THF) the intermediate aza enolates are formed as the
ECCZCN species, as confirmed by spectroscopic
26,39 40 and numerous trapping experiments.
21,22,23 Because of the uniform diastereoface differentiation common for all asymmetric SAMP-/RAMP-hydrazone alkylations, the absolute configuration that will predominate in the final product can be predicted reliably. Furthermore, instead of changing from SAMP to the enantomeric RAMP as chiral auxiliary, it is possible to prepare both enantiomers of target molecules in excess using SAMP, simply by changing the building blocks used as nucleophile and electrophile. This opposite enantioselectivity through synthon control is demonstrated in the cases of
2-methylbutanal,
2-methyloctanal, and
2-methyl-1-octanamine (see Table I).
Another advantage of SAMP-/RAMP-hydrazones is the facile determination of the asymmetric induction by downfield shifting of the SAMP- or RAMP-hydrazone methoxy singlet [LIS-technique, Eu(fod)3].
Besides the oxidative cleavage by ozonolysis, the optically active carbonyl compounds can be alternatively obtained by acidic hydrolysis of the corresponding SAMP-/RAMP-hydrazone methiodides in a two-phase system.
6,8The chiral auxiliary SAMP or RAMP may be recycled by
lithium aluminum hydride reduction of the
nitrosamine (
S)-
5 formed during ozonolysis. Other very successful applications of the SAMP-/RAMP-hydrazone method in natural product synthesis have recently been reported by Nicolaou et al. [ionophore antibiotic X-14547A (indanomycine)]
41 42, Pennanen (eremophilenolide, sesquiterpene),
37 Enders et al. (defensive substance of "daddy longlegs"),
13 Mori et al. (serricornin, cigarette beetle pheromone),
38 and Bestmann et al. (pheromone analogs).
36 Finally, it should be mentioned that the chiral auxiliaries SAMP and RAMP may also be used in the resolution of aldehydes
43 and ketones
44 and in the NMR spectroscopic determination of percent enantiomeric excess ee of chiral aldehydes.
45This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
SAMP-hydrazone (S)-2
3,3-dimethyl-2-butanone-SAMP-hydrazone
benzaldehyde-SAMP-hydrazone
2,2-dimethylpropanal-SAMP-hydrazone
3-Pentanone SAMP hydrazone
(S)-(+)-4-Methyl-3-heptanone SAMP hydrazone
SAMP
RAMP
potassium carbonate (584-08-7)
sulfuric acid (7664-93-9)
HCl (7647-01-0)
Benzene (71-43-2)
ether (60-29-7)
oxygen (7782-44-7)
potassium iodide (7681-11-0)
sodium thiosulfate (7772-98-7)
nitrogen (7727-37-9)
copper (7440-50-8)
Raney nickel (7440-02-0)
iodine (7553-56-2)
Benzophenone (119-61-9)
sodium (13966-32-0)
Catechol (120-80-9)
Diphenylacetic acid (117-34-0)
Pentane (109-66-0)
dichloromethane (75-09-2)
ozone (10028-15-6)
magnesium sulfate (7487-88-9)
borane (7440-42-8)
butyllithium (109-72-8)
(S)-proline (147-85-3)
Tetrahydrofuran (109-99-9)
3-pentanone (96-22-0)
lithium aluminum hydride,
LiAlH4 (16853-85-3)
hexane (110-54-3)
Methyllithium (917-54-4)
nitrosamine (35576-91-1)
argon (7440-37-1)
calcium hydride (7789-78-8)
phosphomolybdic acid (51429-74-4)
lithium diisopropylamide (4111-54-0)
diisopropylamine (108-18-9)
CHLOROTRIMETHYLSILANE (75-77-4)
(S)-1-Amino-2-methoxymethylpyrrolidine (72748-99-3)
Propyl iodide (107-08-4)
2-methylbutanal (96-17-3)
2-methyloctanal (7786-29-0)
2-methyl-1-octanamine
(S)-(+)-4-Methyl-3-heptanone,
3-Heptanone, 4-methyl, (S)- (51532-30-0)
(R)-citronellic acid (18951-85-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved