Org. Synth. 1993, 71, 89
DOI: 10.15227/orgsyn.071.0089
PALLADIUM(0)-CATALYZED REACTION OF 9-ALKYL-9-BORABICYCLO[3.3.1]NONANE WITH 1-BROMO-1-PHENYLTHIOETHENE: 4-(3-CYCLOHEXENYL)-2-PHENYLTHIO-1-BUTENE
Submitted by Tatsuo Ishiyama, Norio Miyaura, and Akira Suzuki
1.
Checked by Ron J. Graham and Leo A. Paquette.
1. Procedure
A. 1-Bromo-1-phenylthioethene. A 300-mL, two-necked, round-bottomed flask is fitted with a magnetic stirring bar, pressure-equalizing dropping funnel, and a reflux condenser to which a nitrogen inlet tube and an oil bubbler are attached, and flushed with nitrogen (Note 1). In the flask are placed 13.6 g (100 mmol) of phenyl vinyl sulfide (Note 2) and 80 mL of ether (Note 3), which are then cooled to ca. −78°C with a dry ice-methanol bath. Bromine (16.0 g, 100 mmol) is added dropwise over 30 min to the stirred solution. After the solution is warmed to room temperature, 40 mL of absolute ethanol, followed by a solution of 8.0 g (140 mmol) of potassium hydroxide in 80 mL of absolute ethanol is added dropwise to the resulting slightly red solution over 30 min. The light brown solution containing a white precipitate of potassium bromide is stirred at room temperature for 2 hr. The precipitate is removed by filtration and the filtrate is concentrated by rotary evaporation. The residue is treated with 200 mL of ether and 200 mL of water. The organic layer is separated, washed with brine (50 mL), and dried over anhydrous magnesium sulfate. After rotary evaporation of the solvent, the residual oil is distilled under reduced pressure to give 17.2 g (80% yield) of 1-bromo-1-phenylthioethene (Note 4) as a colorless liquid, bp 49–50°C (0.07 mm).
B. 9-[2-(3-Cyclohexenyl)ethyl]-9-BBN. A 500-mL, three-necked, round-bottomed flask is equipped with a magnetic stirring bar, thermometer, reflux condenser, and a pressure-equalizing addition funnel capped with a rubber septum. The apparatus is connected through the condenser to a nitrogen source and an oil bubbler and flushed with nitrogen. The flask is charged with 35 mL of tetrahydrofuran (Note 5) and 8.32 g (77.0 mmol) of 4-vinyl-1-cyclohexene (Note 6) and cooled to 0°C. A 0.5 M solution of 9-BBN (9-borabicyclo[3.3.1]nonane) in tetrahydrofuran (154 mL, 77.0 mmol) (Note 7) is transferred via cannula to the addition funnel and is added dropwise with stirring over 1 hr maintaining the temperature at 0–5°C. The reaction mixture is stirred for 1 hr at 0°C and for 1.5 hr at room temperature. The solution obtained is used in the next step without further treatment (Note 8).
C. 4-(3-Cyclohexenyl)-2-phenylthio-1-butene. To the above solution of the borane derivative, 0.809 g (0.700 mmol) of tetrakis(triphenylphosphine)palladium(0) (Note 9), 1.47 g (5.60 mmol) of triphenylphosphine (Note 10), 35 mL of 3 M potassium phosphate in water (Note 11), and finally 15.1 g (70.0 mmol) of 1-bromo-1-phenylthioethene are added and the resulting mixture is heated at reflux for 3 hr with stirring. The light brown solution is cooled to room temperature and treated with 6.4 g (100 mmol) of ethanolamine (Note 12) for 1 hr. Then 100 mL of hexane and 100 mL of water are added. The organic layer is separated, washed with 100 mL of water, and dried over anhydrous magnesium sulfate. The drying agent is removed by filtration and the filtrate is concentrated by rotary evaporation. The addition of 250 mL of hexane to the residual viscous oil, containing some solid, precipitates the 9-BBN/ethanolamine complex. The solid is removed by filtration and is washed with hexane (50 mL × 3), and the filtrate is concentrated using a rotary evaporator. The crude product is passed through a short silica gel column (60–200 mesh, 60 g) using hexane as an eluent (Note 13). After removal of the hexane, the residue is distilled under reduced pressure to give 12.5–13.9 g (73–81%) of 4-(3-cyclohexenyl)-2-phenylthio-1-butene as a colorless liquid, bp 114–116°C (0.04 mm) (Note 14).
2. Notes
1.
All glassware was pre-dried in an
oven at 120°C for 2 hr, assembled while hot, and allowed to cool under a stream of
nitrogen.
3.
Ether was distilled from
benzophenone ketyl under
nitrogen before use.
4.
The product is labile at room temperature and should be stored in a freezer. Spectral data are as follows: IR (neat) cm
−1: 3076, 3060, 1583, 1477, 1440, 1069, 752, 689;
1H NMR (300 MHz, CDCl
3) δ: 5.73 (d, 1 H, J = 2.3), 5.83 (d, 1 H, J = 2.3), 7.23–7.46 (m, 5 H).
5.
Tetrahydrofuran is distilled from
benzophenone ketyl under
nitrogen before use.
6.
4-Vinyl-1-cyclohexene was obtained from Aldrich Chemical Company, Inc., and distilled it prior to use.
7.
A 0.5 M solution of
9-BBN in tetrahydrofuran was purchased from Aldrich Chemical Company, Inc., and was used without additional purification. The preparation
2 of the reagent by hydroboration of
1,5-cyclooctadiene with
borane/
tetrahydrofuran complex is reported.
8.
If necessary,
9-[2-(3-cyclohexenyl)ethyl]-9-BBN3 can be purified by removal of the solvent and vacuum distillation under
nitrogen [bp
103°C (0.035 mm)].
9.
The preparation of
tetrakis(triphenylphosphine)palladium(0) is described.
4 It is also available from Aldrich Chemical Company, Inc.
10.
Triphenylphosphine was obtained from Nakarai Chemicals, Japan. When the reaction is carried out without additional
triphenylphosphine, the yield of coupling product may drop to 60–70% and the product is accompanied by the by-products,
phenyl vinyl sulfide and
4-vinyl-1-cyclohexene, derived from β-hydride elimination.
11.
The solution is prepared by dissolving
22.3 g (105 mmol) of potassium phosphate (Nakarai Chemicals, Japan) in water and adjusting the final volume to 35 mL. The original method
5 used
sodium hydroxide as base;
potassium phosphate is desirable for the extension of the present procedure to base-sensitive compounds. Under such conditions, the reaction with
9-(10-carbomethoxydecanyl)-9-BBN proceeds similarly without saponification of the ester group.
12.
Ethanolamine was purchased from Nakarai Chemicals, Japan. The reagent reacts with the resulting 9-BBN residue to give an air stable 1:1 adduct
6 that is insoluble in
hexane.
13.
This operation effectively removes the remaining palladium-containing compounds, phosphine derivatives, and borane residues.
14.
Gas chromatographic analysis of the product (Finnigan ITD 800-fused silica capillary, SE 30 column, 0.35 mm × 25 m, column temperature 80–250°C, injection temperature 250°C) shows that the chemical purity is 94–98.5%. The spectral data are as follows: IR (neat) cm
−1: 3030, 2920, 1615, 1590, 1480, 1440, 750, 690;
1H NMR (300 MHz, CDCl
3) δ: 1.00–1.90 (m, 6 H), 1.90–2.20 (m, 3 H), 2.20–2.50 (m, 2 H), 4.88 (s, 1 H), 5.15 (s, 1 H), 5.64 (s, 2 H), 7.20–7.50 (m, 5 H). The product deteriorates at room temperature and should be stored in the freezer.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The reaction described here is a method for the synthesis of alkenyl sulfides via the palladium-catalyzed cross-coupling reaction of 9-alkyl-9-BBN.
5 Bromo(phenylthio)ethene has several advantages in terms of its practical use for the cross-coupling reaction. The coupling occurs at the
bromine position, but no coupling products at the
sulfur position are obtained even under conditions using an excess of 9-alkyl-9-BBN. Thus, the formation of dialkylation products is completely avoided. The reaction is highly stereoselective and readily extended to the coupling with (E)- and (Z)-2-bromo-1-phenylthio-1-alkenes (
1 and
2).
7 The reactions of (E)-, (Z)-1-alkenyl, or 1,3-alkadienylboronic esters with
1 or
2 provide simple routes for the stereoselective syntheses of 1,3-alkadienyl and 1,3,5-alkatrienyl phenyl sulfides.
8 Another route to vinylic sulfides involves the cross-coupling of 1-alkenyl halides with lithium, tin, and boron thioalkoxides.
9 These routes are convenient when the corresponding alkenyl halides are easily available.
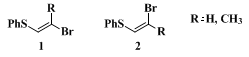
The ready availability of 2-(organothio)-1-alkenylboron compounds, obtained by the catalytic hydroboration of 1-organothio-1-alkynes (eq. 1) or the thioboration of 1-alkynes (Scheme 1), may offer other flexible and reliable routes to such stereodefined alkenyl sulfides in combination with the cross-coupling reaction with organic halides. The catalytic hydroboration of thioalkynes with
catecholborane in the presence of
NiCl2(dppe) or
PdCl2(dppf) regio- and stereoselectively gives
3.
10The sequential catalytic hydroboration and cross-coupling reactions with a common
palladium catalyst allows the one-pot synthesis of 1-alkenyl sulfides (eq. 1).
11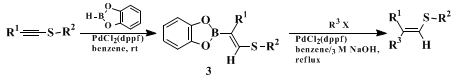
When a solution of terminal alkyne and 9-RS-9-BBN in
THF is heated at 50°C for 3 hr in the presence of
Pd(PPh3)4 (3 mol %), the cis-addition of the B-S bond to alkyne proceeds regio- and stereoselectively (Scheme 1).
12 The adduct
4 is susceptible to C-B bond breaking or stereochemical isomerization, but the in situ preparation and subsequent cross-coupling with organic halides gives a variety of alkenyl sulfides retaining the original configuration of
4. The vinylboranes thus obtained have unusually high nucleophilicity due to the activation by an electron-donating β-organothio group. The protodeboronation proceeds instantaneously with
methanol to provide the thiol adducts. The addition to aldehydes and the Michael addition to α,β-unsaturated ketones or aldehydes at the refluxing temperature of THF afford various vinyl sulfides.
13The synthesis of vinylic sulfides via the cross-coupling reaction of organoboron compounds and other related reactions were recently reviewed.
14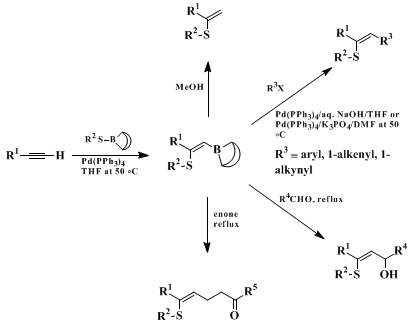
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
benzophenone ketyl
brine
9-BBN
9-[2-(3-Cyclohexenyl)ethyl]-9-BBN
9-BBN (9-borabicyclo[3.3.1]nonane)
9-(10-carbomethoxydecanyl)-9-BBN
NiCl
PdCl
Pd(PPh3)4
ethanolamine complex
ethanol (64-17-5)
methanol (67-56-1)
ether (60-29-7)
sodium hydroxide (1310-73-2)
bromine (7726-95-6)
nitrogen (7727-37-9)
sulfur (7704-34-9)
potassium hydroxide (1310-58-3)
palladium (7440-05-3)
potassium bromide (7758-02-3)
magnesium sulfate (7487-88-9)
ethanolamine (141-43-5)
borane (7440-42-8)
Tetrahydrofuran,
THF (109-99-9)
hexane (110-54-3)
triphenylphosphine (603-35-0)
1,5-cyclooctadiene
4-vinyl-1-cyclohexene
CATECHOLBORANE (274-07-7)
tetrakis(triphenylphosphine)palladium(0) (14221-01-3)
Phenyl vinyl sulfide (1822-73-7)
1-BROMO-1-PHENYLTHIOETHENE (80485-53-6)
4-(3-Cyclohexenyl)-2-phenylthio-1-butene (155818-88-5)
Bromo(phenylthio)ethene
potassium phosphate (7778-53-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved