Org. Synth. 1997, 74, 248
DOI: 10.15227/orgsyn.074.0248
REGIOSELECTIVE SYNTHESIS OF 3-SUBSTITUTED INDOLES: 3-ETHYLINDOLE
[1H-Indole, 3-ethyl-]
Submitted by Mercedes Amat, Sabine Hadida, Swargam Sathyanarayana, and Joan Bosch
1.
Checked by Ji Liu, Chris H. Senanayake, and Ichiro Shinkai.
1. Procedure
Caution! tert-Butylithium is extremely pyrophoric and must not be allowed to come into contact with the atmosphere. This reagent should only be handled by individuals trained in its proper and safe use. It is recommended that transfers be carried out by using a 20-mL or smaller glass syringe filled to no more than 2/3 capacity, or by cannula. For a discussion of procedures for handling air-sensitive reagents, see Aldrich Technical Bulletin AL-134. [Note added August 2009]
A. 3-Bromo-1-(tert-butyldimethylsilyl)indole. An oven-dried, 500-mL, three-necked, round-bottomed flask, equipped with a magnetic stirring bar, 100-mL pressure-equalizing addition funnel, and an argon inlet and outlet, is charged with indole (8.0 g, 0.068 mol) (Note 1) and tetrahydrofuran (200 mL) (Note 2). The solution is stirred and cooled to −78°C with a dry ice/acetone bath, and a solution of butyllithium in hexane (47 mL of a 1.6 M solution, 0.075 mol) (Note 3) is added dropwise via cannula. The mixture is warmed to −10°C, stirred for 15 min, and cooled to −50°C. A solution of tert-butyldimethylsilyl chloride (11.6 g, 0.077 mol) (Note 3) in tetrahydrofuran (60 mL) is added dropwise to this mixture. The temperature is raised to 0°C and after 3 hr the reaction mixture is cooled to −78°C. Freshly crystallized N-bromosuccinimide (12.18 g, 0.0684 mol) (Note 4) is added via a solid-addition funnel and the resulting mixture is stirred in the dark at −78°C for 2 hr and allowed to warm to room temperature. Hexane (100 mL) and pyridine (1 mL) are added and the resulting suspension is filtered through a Celite pad. The filtrate is evaporated under reduced pressure. The crude residue is purified (Note 5) by flash chromatography on silica gel (Note 6) (350 g, 30 cm × 6 cm) (100% hexane) to give 17.8 g (84%) of 3-bromo-1-(tert-butyldimethylsilyl)indole as a colorless solid (Note 7).
B. 1-(tert-Butyldimethylsilyl)-3-ethylindole. An oven-dried, 500-mL, three-necked, round-bottomed flask, equipped with a magnetic stirring bar, 50-mL pressure-equalizing addition funnel, and an argon inlet and outlet, is charged with 3-bromo-1-(tert-butyldimethylsilyl)indole (10 g, 0.032 mol) and tetrahydrofuran (100 mL). The mixture is stirred and cooled to −78°C with a dry ice/acetone bath. A solution of tert-butyllithium (Note 8) (41.7 mL of a 1.7 M solution in pentane, 0.071 mol) is transferred slowly to the above mixture from a graduated tube via a stainless steel cannula under positive argon pressure. The reaction mixture becomes yellow. Stirring is continued at −78°C for 10 min. A solution of ethyl iodide (5.2 mL, 0.065 mol) (Note 9) in tetrahydrofuran (20 mL) is added dropwise over 15 min to the resulting 1-(tert-butyldimethylsilyl)-3-lithioindole. The reaction mixture becomes colorless, and after 15 min it is allowed to reach room temperature, poured into a cold saturated sodium carbonate solution (200 mL), and extracted with methylene chloride (3 × 100 mL). The combined organic layers are washed with water (100 mL), dried over sodium sulfate, and evaporated under reduced pressure to give 8.0 g (96%) of 1-(tert-butyldimethylsilyl)-3-ethylindole as a light pink oil (Note 10).
C. 3-Ethylindole. An oven-dried, 250-mL, round-bottomed flask, equipped with a magnetic stirring bar, rubber septum, and an argon inlet and outlet, is charged with 1-(tert-butyldimethylsilyl)-3-ethylindole (8 g, 0.031 mol) and tetrahydrofuran (100 mL). The mixture is stirred and a 1 M solution of tetrabutylammonium fluoride (TBAF) in tetrahydrofuran (31 mL, 0.031 mol) (Note 11) is added. After the solution is stirred for 10 min at room temperature, it is poured into a saturated solution of sodium carbonate (200 mL) and extracted with dichloromethane (3 × 100 mL). The combined organic layers are washed with water (100 mL), dried over sodium sulfate, and evaporated under reduced pressure. The residue is subjected to flash chromatography on silica gel (175 g, 32 cm × 4.5 cm) (25% dichloromethane-hexane, v/v) to give 4.1 g (92%) of 3-ethylindole as colorless plates (Note 12).
2. Notes
1.
Indole was obtained from Fluka Chemie AG, and was crystallized from
hexane and dried over
phosphorus pentoxide (P2O5) before use.
2.
Tetrahydrofuran was distilled from
sodium benzophenone ketyl immediately before use.
3.
Butyllithium and tert-butyldimethylsilyl chloride were obtained from Fluka Chemie AG and used as received.
4.
N-Bromosuccinimide was obtained from Fluka Chemie AG, and crystallized from water and dried over
P2O5 before use.
5.
Chromatographic purification of the reaction mixture must be effected as soon as possible after workup in order to separate traces of contaminating
3-bromoindole, which promotes rapid decomposition. Pure
3-bromo-1-(tert-butyldimethylsilyl)indole can be stored under
argon at 4°C without appreciable decomposition.
6.
Silica gel (35–70 mesh) was used as received.
7.
The spectral properties for
3-bromo-1-(tert-butyldimethylsilyl)indole are as follows:
1H NMR (300 MHz, CDCl
3) δ: 0.60 (s, 6 H), 0.93 (s, 9 H), 7.17 (s, 1 H), 7.20 (m, 2 H), 7.48 (m, 1 H), 7.54 (m, 1 H);
13C NMR (75 MHz, CDCl
3) δ: −4.0 (CH
3Si), 19.3 [C(CH
3)
3], 26.2 [C(CH
3)
3], 93.6 (C-3), 114.0 (C-7), 119.1 (C-4), 120.5 (C-5), 122.5 (C-6), 129.6 (C-2), 129.8 (C-3a), 140.2 (C-7a). Anal Calcd for C
14H
20BrNSi: C, 54.18; H, 6.50; Br, 25.75; N, 4.51. Found: C, 54.21; H, 6.60; Br, 25.52; N, 4.62. Attempts to crystallize
3-bromo-1-(tert-butyldimethylsilyl)indole were unsuccessful.
8.
tert-Butyllithium (1.7 M solution in pentane) was obtained from Aldrich Chemical Company, Inc., and used as received.
9.
Ethyl iodide was obtained from Fluka Chemie AG and distilled prior to use.
10.
NMR spectrum shows the presence of less than
3% of 1-(tert-butyldimethylsilyl)indole. Crude
1-(tert-butyldimethylsilyl)-3-ethylindole can be purified by flash column chromatography on
silica gel (35-70 mesh) (350 g, 30 cm × 6 cm) (
100% hexane) to give pure
1-(tert-butyldimethylsilyl)-3-ethylindole as a colorless oil in about
90% yield. The spectral properties are as follows:
1H NMR (300 MHz, CDCl
3) δ: 0.57 (s, 6 H), 0.92 (s, 9 H), 1.32 (t, 3 H, J = 7.5), 2.76 (g, 2 H, J = 7.5), 6.92 (s, 1 H), 7.12 (m, 2 H), 7.47 (m, 1 H), 7.58 (m, 1 H);
13C NMR (75 MHz, CDCl
3) δ: −3.9 (CH
3Si), 14.5 (CH
3CH
2), 18.4 (CH
2CH
3), 19.5 [C(CH
3)
3], 26.3 [C(CH
3)
3], 113.8 (C-7), 118.8 (C-4), 119.2 (C-5), 119.8 (C-3), 121.3 (C-6), 126.9 (C-2), 130.9 (C-3a), 141.6 (C-7a). Anal. Calcd for C
16H
25NSi: C, 74.06; H, 9.71; N, 5.40. Found: C, 74.18; H, 9.73; N, 5.46.
11.
Tetrabutylammonium fluoride in tetrahydrofuran (1 M solution) was obtained from Fluka Chemie AG and used as received.
12.
The spectral properties for
3-ethylindole are as follows:
1H NMR (200 MHz, CDCl
3) δ: 1.37 (t, 3 H, J = 7.5), 2.82 (q, 2 H, J = 7.5), 6.98 (br s, 1 H), 7.18 (m, 2 H), 7.37 (d, 1 H, J = 7.5), 7.66 (d, 1 H, J = 7.8), 7.85 (br s, 1 H);
13C NMR (50 MHz, CDCl
3) δ: 15.0 (CH
3CH
2), 18.9 (CH
2CH
3), 111.6 (C-7), 119.3 (C-3), 119.5 (C-4), 119.6 (C-5), 121.1 (C-6), 122.4 (C-2), 128.0 (C-3a), 136.9 (C-7a).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Although there are many studies about the preparation and synthetic applications of N-protected 2-lithioindoles,
2 their isomers, the 3-lithioindoles, have received little attention. Thus, the only indole protecting group used for the preparation of simple 3-lithioindoles is the benzenesulfonyl group.
1-(Benzenesulfonyl)-3-lithioindole is prepared at −100°C by halogen-metal exchange with
tert-butyllithium from the corresponding 3-iodo-
3 or
3-bromoindole.
4 At higher temperatures it rearranges to the thermodynamically more stable 2-lithio isomer. On the other hand, some 2-substituted 1-(benzenesulfonyl)-3-lithioindoles undergo ring fragmentation to give 2-aminophenylacetylene derivatives.
5 The change of the benzenesulfonyl protecting group for a trialkylsilyl group allows the preparation of 3-lithioindoles that are relatively stable species even at room temperature.
In the present procedure,
indole is protected with a tert-butyldimethylsilyl group and further brominated with
N-bromosuccinimide in a one-pot reaction.
3-Bromo-1-(tert-butyldimethylsilyl)indole readily undergoes halogen-lithium exchange with
tert-butyllithium at −78°C. Subsequent reaction with an electrophile is exemplified by the reaction with
ethyl iodide to give
1-(tert-butyldimethylsilyl)-3-ethylindole. Other electrophiles such as alkyl or allyl halides,
ethylene oxide, acylating reagents,
carbon dioxide, aromatic aldehydes, and
trimethyltin chloride have also been employed successfully in a 500 mg scale (see Table).
6 The reactions regioselectively lead to 3-substituted indoles. 2-Substituted indoles were not detected, indicating that
1-(tert-butyldimethylsilyl)-3-lithioindole (1) does not undergo rearrangement to the 2-lithio isomer. Products arising from a ring fragmentation were not detected either. When the reactions of
1 with
ethyl iodide and
methyl iodide were carried out at room temperature, the yields of the respective 3-alkylindoles were similar to those obtained when operating at −78°C. Finally, the 1-tert-butyldimethylsilyl protecting group can be readily removed by treatment with TBAF under mild conditions.
TABLE
SYNTHESIS OF 3-SUBSTITUTED INDOLES
|
|
Entry
|
Electrophile
|
R
|
2 Yield(%)
|
3Yield(%)a
|
|
|
a
|
MeI
|
Me
|
90
|
85
|
|
b
|
BuBr
|
Bu
|
64
|
61
|
|
c
|
(CH2CH2)O
|
CH2CH2OH
|
63
|
62
|
|
d
|
Me2C=CHCH2Brb
|
CH2CH=CMe2
|
|
69c
|
|
e
|
HCONMe2d
|
CHO
|
e
|
94
|
|
f
|
C6H5COCld
|
COC6H5
|
e
|
84
|
|
g
|
C6H5CO2CH3d
|
COC6H5
|
69
|
58
|
|
h
|
ClCO2CH3d
|
CO2CH3
|
84
|
80
|
|
i
|
CO2
|
CO2H
|
94
|
70f
|
|
j
|
C6H5CHO
|
CHOHC6H5
|
67
|
g
|
|
k
|
4-CHO-C5H4N
|
4-CHOH-C5H4N
|
55
|
g
|
|
l
|
ClSnMe3
|
SnMe3
|
94
|
h
|
|
aOverall yield after purification by column chromatography. bThe 3-lithioindole 1 was converted into a cuprate by addition of 1 equiv of CuBr·SMe2. cAn 85:15 mixture of 3d and the isomer in which R is CMe2CH=CH2, respectively, is obtained. dThe best yields were obtained by reverse addition of the lithium derivative 1 to a THF solution of the electrophile at −78°C. eThe corresponding 3-acyl derivatives undergo partial desilylation during the work-up. fThis desilylation was best effected by using CsF instead of TBAF. gThese carbinols were obtained as pink oils, which partially decomposed during purification by column chromatography. hAttempts to deprotect the tin derivative 2l afforded only indole.
|
The triisopropylsilyl group gave comparable satisfactory results.
7As a further synthetic application,
3-lithioindole 1 was converted to
1-(tert-butyldimethylsilyl)-3-indolylzinc chloride, which has been successfully employed in the heteroarylation of the
indole 3-position by a
palladium(0)-catalyzed cross-coupling reaction.
8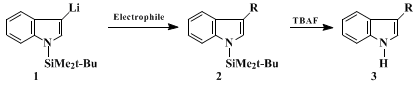
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
silica gel
P2O5
sodium benzophenone ketyl
sodium carbonate (497-19-8)
sodium sulfate (7757-82-6)
carbon dioxide (124-38-9)
pyridine (110-86-1)
palladium (7440-05-3)
Ethylene oxide (75-21-8)
Methyl iodide (74-88-4)
Pentane (109-66-0)
methylene chloride,
dichloromethane (75-09-2)
Ethyl iodide (75-03-6)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
N-bromosuccinimide (128-08-5)
Indole (120-72-9)
hexane (110-54-3)
argon (7440-37-1)
Tetrabutylammonium fluoride (429-41-4)
phosphorus pentoxide (1314-56-3)
tert-Butyllithium (594-19-4)
trimethyltin chloride (1066-45-1)
tert-butyldimethylsilyl chloride (18162-48-6)
3-Ethylindole,
1H-Indole, 3-ethyl- (1484-19-1)
3-Bromo-1-(tert-butyldimethylsilyl)indole (153942-69-9)
1-(tert-Butyldimethylsilyl)-3-ethylindole (153942-71-3)
1-(tert-butyldimethylsilyl)-3-lithioindole
3-bromoindole
1-(tert-butyldimethylsilyl)indole
1-(Benzenesulfonyl)-3-lithioindole
3-lithioindole
1-(tert-butyldimethylsilyl)-3-indolylzinc chloride
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved