Org. Synth. 1996, 73, 116
DOI: 10.15227/orgsyn.073.0116
A SIMPLE AND CONVENIENT METHOD FOR THE OXIDATION OF ORGANOBORANES USING SODIUM PERBORATE: (+)-ISOPINOCAMPHEOL
[Bicyclo[3.1.1]heptan-3-ol, 2,6,6-trimethyl-, [1S-(1α,2β,3α,5α)]-]
Submitted by George W. Kabalka, John T. Maddox, Timothy Shoup, and Karla R. Bowers
1.
Checked by C. Huart and Leon Ghosez.
1. Procedure
CAUTION! This procedure should be conducted in an efficient fume hood to assure the adequate removal of hydrogen, a flammable gas which forms explosive mixtures with air.
A.
(+)-Diisopinocampheylborane. A
dry, 250-mL, three-necked, round-bottomed flask, equipped for magnetic stirring, and with a nitrogen inlet vented through a mercury bubbler, a rubber septum, and a thermometer, is flushed with
nitrogen and charged with
13.75 g (0.101 mol) of (−)-α-pinene and
25 mL of tetrahydrofuran (Note 1),
(Note 2),
(Note 3)). The mixture is cooled to 0°C in an
ice-water bath, magnetic stirring is initiated, and
58.0 mL (0.055 mol) of 0.95 M borane-tetrahydrofuran solution (Note 4) is added via syringe at a rate such that the temperature of the reaction mixture remains below 5°C. After the addition is complete, the cooling bath is removed. The reaction mixture is allowed to warm to room temperature and stir for 2 hr to ensure complete reaction.
2
B.
(+)-Isopinocampheol. To the stirred solution of
(+)-diisopinocampheylborane in tetrahydrofuran, prepared above, 50 mL of distilled water is
slowly added dropwise via syringe [
CAUTION!]
(Note 5) followed by the slow addition of
16.41 g (0.107 mol) of solid sodium perborate tetrahydrate (Note 6) through an appropriate addition funnel at a rate such that the temperature of the reaction mixture does not exceed 35°C
(Note 7). Stirring is continued at room temperature (22°C) for 2 hr to ensure completion of the oxidation reaction. The contents of the flask are then poured into 70 mL of ice-cold water in a
separatory funnel. After thorough mixing, the organic layer is removed and the aqueous layer is extracted twice with
25 mL of ether. The combined
ether extracts are washed twice with 20-mL portions of water and then with
50 mL of saturated aqueous sodium chloride solution. The ether layer is dried over
anhydrous magnesium sulfate, filtered, and concentrated on a
rotary evaporator. The crude product is purified by short-path vacuum distillation to give
14.1–14.3 g (
91–92%) of
isopinocampheol, bp
68°C (0.7 mm), as white needles (mp
53–55°C) that crystallize in the receiving flask
(Note 8). Recrystallization from
pentane gives pure
isopinocampheol as needles, mp
54–55°C (uncorrec.), [α]
25D 34.4° (
benzene,
c 10) indicating 96.4% enantiomeric purity based on the highest reported literature value of 35.7°
(Note 9).
3
2. Notes
1.
All glassware was predried at 140°C for at least 4 hr, assembled hot, flame dried, and cooled under a stream of
nitrogen.
2.
(1S)-(−)-α-Pinene [99%, 98% optical purity, [α]25D −50.6° neat] was purchased from Aldrich Chemical Company, Inc., and distilled under reduced pressure from
lithium aluminum hydride before use.
23.
Tetrahydrofuran was distilled under
nitrogen from
sodium benzophenone ketyl.
4.
Borane-tetrahydrofuran complex (1.0 M) was obtained from Aldrich Chemical Company, Inc., and the concentration of the solution determined using the literature procedure.
45.
Since the hydroboration only proceeds to the dialkylborane stage, a large amount of hydrogen is evolved on hydrolysis. Very slow dropwise addition of water and adequate ventilation are recommended.6.
Sodium perborate tetrahydrate was purchased from Aldrich Chemical Company, Inc. and used as received.
7.
During the addition of
sodium perborate the reaction flask is kept in a
water bath (25°C).
8.
An
air condenser is employed for the distillation. The receiving flask is immersed in an
ice bath.
9.
The product exhibits the following spectral properties: IR (melt) cm
−1: 3300 (OH), 2930, 1472, 1450, 1384, 1367, 1050, 1015;
1H NMR (250 MHz, CDCl
3) δ: 0.92 (s, 3 H), 1.04 (d, 1 H, J = 9), 1.13 (d, 3 H, J = 7), 1.22 (s, 3 H), 1.67–2.11 (m, 5 H), 2.30–2.58 (m, 2 H), 4.06 (dt, 1H);
13C NMR (62.87 MHz, CDCl
3) δ: 20.7, 23.7, 27.7, 34.4, 38.1, 39.1, 41.8, 47.8, 47.9, 71.7.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
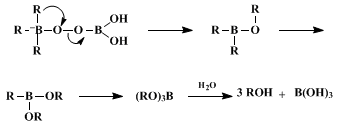
This procedure illustrates the simplest and most convenient method for oxidizing organoboranes, in the present example a dialkylborane. It uses
sodium perborate,
5 an inexpensive, safe, and easily handled reagent, as the oxidizing agent. The reaction proceeds under mild conditions and the yield of the product alcohol is generally as high or higher than that obtained in the sodium hydroxide/hydrogen peroxide oxidation procedure.
6 Thus, the
sodium perborate method is an attractive alternative to the base/hydrogen peroxide oxidation procedure. In the case described above, the perborate procedure produces higher yields of
(−)-isopinocampheol than the sodium hydroxide/hydrogen peroxide procedure with comparable stereoselectivity.
7 Although the mechanism of the oxidation has not been investigated in detail,
sodium perborate does not appear to be acting as a simple mixture of
hydrogen peroxide and
sodium borate.
8,9,10,11 Presumably, borate is a more effective leaving group (Scheme 1) than hydroxide ion which is generated during oxidation by
hydrogen peroxide .
Sodium perborate, owing to its stability, commercial availability, and ease of handling, should prove to be a popular reagent for oxidizing organoboranes. Some representative examples of the oxidation of organoboranes bearing a variety of alkyl and aryl groups are listed in Table I.
12 13
TABLE I
SODIUM PERBORATE OXIDATION OF ORGANORANESa
|
|
Alkene
|
Reagent
|
Product
|
Yield(%)b
|
|
|
|
|
|
93
|
|
|
|
|
99
|
|
|
BH3
|
|
92
|
|
|
BH3
|
|
86
|
|
|
|
|
84
|
|
|
|
|
87
|
|
|
aThe organoboranes were formed via the hydroboration of the alkene listed. bIsolated yield. cOne equivalent of 2,3-dimethyl-2-butanol was isolated in addition to 2 equiv of 2-hexanol. dOne equivalent of 1,4-cyclooctanediol was isolated in addition to the 1-hexanol. eTriphenylborane was purchased from Aldrich Chemical Company, Inc.
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
( − )-α-pinene
sodium benzophenone ketyl
(−)-ISOPINOCAMPHEOL
(1S)-(−)-α-Pinene
Benzene (71-43-2)
ether (60-29-7)
hydrogen (1333-74-0)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
hydrogen peroxide (7722-84-1)
Pentane (109-66-0)
magnesium sulfate (7487-88-9)
borane (7440-42-8)
Tetrahydrofuran (109-99-9)
lithium aluminum hydride (16853-85-3)
sodium borate
sodium perborate tetrahydrate (10486-00-7)
Isopinocampheol,
(+)-Isopinocampheol (27779-29-9)
(+)-Diisopinocampheylborane (21947-87-5)
SODIUM PERBORATE
Bicyclo[3.1.1]heptan-3-ol, 2,6,6-trimethyl-, [1S-(1α,2β,3α,5α)]- (24041-60-9)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved