Org. Synth. 1999, 76, 110
DOI: 10.15227/orgsyn.076.0110
PREPARATION OF ENANTIOMERICALLY PURE α-N,N-DIBENZYLAMINO ALDEHYDES: S-2-(N,N-DIBENZYLAMINO)-3-PHENYLPROPANAL
[
Benzenepropanal, α-[bis(phenylmethyl)amino]-, (S)-
]
Submitted by Manfred T. Reetz
1
, Mark W. Drewes
2
, and Renate Schwickardi
1
.
Checked by Yan Dong, Anthony Laurenzano, and Steven Wolff.
1. Procedure
Caution! Lithium aluminum hydride is a flammable solid, that upon contact with moisture, forms hydrogen, a highly flammable and potentially explosive gas.
A. Benzyl (S)-2-(N,N-dibenzylamino)-3-phenylpropanoate. A 250-mL, three-necked, round-bottomed flask, equipped with a magnetic stirring bar, a reflux condenser, and a dropping funnel, is charged with a solution of
16.6 g (120 mmol) of potassium carbonate
and
4.8 g (120 mmol) of sodium hydroxide
in 100 mL of water. Following the addition of
9.9 g (60 mmol) of S-phenylalanine
(Note 1), the stirred suspension is heated at reflux to form a clear solution. To this refluxing solution is added dropwise
31.0 g (181 mmol) of distilled benzyl bromide
. The mixture is heated at reflux for an additional 1 hr and then cooled to room temperature. The organic phase is separated, and the aqueous phase is extracted twice with
75 mL of diethyl ether
. The combined organic phases are washed with about
75 mL of a saturated aqueous solution of sodium chloride (NaCl) and dried over
magnesium sulfate (MgSO4). Following the removal of the solvent under reduced pressure, the crude product is purified using flash chromatography (
250 g of 230-400 mesh silica gel
;
hexane/ethyl acetate
10:1, v/v) to provide 15.14-18.0 g (58-69%) of
benzyl (S)-2-(N,N-dibenzylamino)-3-phenylpropanoate
(Note 2).
B. (S)-2-(N,N-Dibenzylamino)-3-phenyl-1-propanol. A dry, 100-mL, three-necked, round-bottomed flask, equipped with a magnetic stirring bar and a dropping funnel, is charged with
60 mL of dry diethyl ether
and
1.13 g (30 mmol) of lithium aluminum hydride
(Note 3) under an atmosphere of argon
(Note 4). The suspension is cooled to 0°C, and
10.9 g (25 mmol) of benzyl (S)-2-(N,N-dibenzylamino)-3-phenylpropanoate in 10 mL of diethyl ether
are added dropwise. The mixture is stirred for 16 hr at room temperature and cooled again to 0°C. The mixture is carefully worked up by the dropwise and sequential addition of 1.1 mL of water,
1.1 mL of a 15% aqueous sodium hydroxide
solution and an additional 3.4 mL of water. The reaction mixture is filtered through a coarse filtration frit to remove aluminum salts, and the latter are washed four times with
8-mL portions of diethyl ether
. The combined filtrates and washings are dried over magnesium sulfate and concentrated under reduced pressure. Most of the
benzyl alcohol
is removed under high vacuum (10−5 mbar, 0.75 × 10−5 mm) at a bath temperature of 50°-60°C. The crude product is then purified by flash chromatography (
100 g of 230-400 mesh silica gel
; hexane/ethyl acetate 95:5, v:v) or recrystallized from hexane to afford 6.2-7.2 g (75-87% yield) of a white solid (Note 5) having a melting point of 67°C and an optical rotation of [α]20
D
+35.6° (
CH2Cl2
, c 1.91). The enantiomeric purity as measured by HPLC [Varian 5560; chiral stationary phase: 250 mm Chiralcel OD-H, 4.6 mm i. d.; mobile phase:
heptane
:
2-propanol
= 90 : 10; T/p/F: 308 K/3.2 Mpa/0.5 mL/min; sample volume: 5 μL (2.3 mg in
0.5 mL of heptane
,
0.05 mL of 2-propanol
); detector: UV 200, 254 nm, TC = 0.05 sec. E = 0.1] is >99% (Note 6).
C.
(S)-2-(N,N-Dibenzylamino)-3-phenylpropanal
. This procedure is based on the Swern oxidation
3
(Note 7). A dry, 250-mL, three-necked, round-bottomed flask equipped with a
magnetic stirrer and a dropping funnel is charged with
100 mL of dry dichloromethane
under an atmosphere of
argon
(Note 3). After cooling to −78°C,
1.52 g (12 mmol) of oxalyl chloride
(Note 4) and
1.56 g (20 mmol) of dry dimethyl sulfoxide
are added dropwise to the stirred solution. After 5 min,
3.31 g (10 mmol) of (S)-2-(N,N-dibenzylamino)-3-phenyl-1-propanol in 5 mL of dichloromethane
are added with stirring. The mixture is stirred for an additional 0.5 hr at −78°C, and
4.05 g (40 mmol) of freshly distilled triethylamine
is added. The mixture is then allowed to warm to room temperature over 0.5 hr, whereupon 50 mL of water is added. The phases are separated, and the aqueous phase is extracted three times with
50 mL of diethyl ether
or
dichloromethane. The combined organic phases are washed successively with
10 mL of aqueous 1%
hydrochloric acid
, 10 mL of water,
10 mL of aqueous 5%
sodium bicarbonate
, and
10 mL of saturated aqueous NaCl
. The organic layer is dried over
MgSO4
, and the solvent is removed under reduced pressure, to provide
3.22 g (
95-98% yield) of the aldehyde as a crude product having a purity of ≥95%
(Note 8) and
(Note 9).
2. Notes
1.
S-Phenylalanine was purchased from Aldrich Chemical Company, Inc.
, and used without further purification.
2.
The product has the following spectral properties:
1H NMR (400 MHz, CDCl
3) δ: 3.00 and 3.14 (ABX System, 2 H, J
AB = 14.1, J
AX = 7.3 and J
BX = 5.9, CH
2-CH-N), 3.54 and 3.92 (AB System, 4 H, J
AB = 13.9, N-CH
2), 3.71 (t, 1 H, J = 7.6, CH
2-CH-N), 5.11 and 5.23 (AB System, 2 H, J
AB = 12.3, O-CH
2-C
6H
5) and 7.18 (m, 20 H, O-CH
2-C
6
H
5, N-CH
2-C
6
H
5 and C
6
H
5-CH
2)
;
13C NMR (100 MHz, CDCl
3) δ: 35.8 (t, C
6H
5-CH
2), 54.5 (t, N-CH
2), 62.5 (d, CH
2-CH-N), 66.0 (t, O-CH
2-C
6H
5), 126.3-139.3 (m, O-CH
2-C
6
H
5, N-CH
2-C
6
H
5 and C
6
H
5-CH
2) and 172.1 (s, COO-CH
2)
. Anal. Calcd for C
30H
29NO
2: C, 82.72; H, 6.72; N, 3.22. Found: C, 82.86; H, 6.73; N, 3.50.
3.
Nitrogen can also be used as the inert gas.
4.
Oxalyl chloride was purchased from Merck, Darmstadt/Germany or Aldrich Chemical Company, Inc.
and used as received.
5.
The product has the following spectral properties:
1H NMR (400 MHz, CDCl
3) δ: 2.36-2.50 (m, 1 H, CH-C
6H
5), 3.30-3.58 (m, 2 H), 3.29-3.38 (m, 1 H), 3.48 and 3.92 (AB System, 4 H, J
AB = 13.3, N-CH
2), 3.45-3.52 (m, 1 H) and 7.06-7.36 (m, 15 H, N-CH
2-C
6
H
5 and C
6
H
5-CH
2)
;
13C NMR (100 MHz, CDCl
3) δ: 31.9 (t, C
6H
5-CH
2), 53.4 (t, N-CH
2), 60.6 (d, CH
2-CH-N), 61.1 (t, CH
2-OH) and 126.2-134.3 (m, N-CH
2-C
6
H
5 and C
6
H
5-CH
2)
. The signal for the hydroxy
hydrogen is centered at δ = 2.97 and is very broad. Anal. Calcd for C
23H
25NO: C, 83.35; H, 7.60; N, 4.23. Found: C, 83.25; H, 7.65; N, 4.20.
6.
An alternative synthesis of this compound which avoids
LiAlH4
involves N-benzylation of commercially available
(e. g., Aldrich Chemical Company, Inc.) (S)-2-amino-3-phenylpropanol
as follows: A mixture of
3.78 g (25 mmol) of (S)-2-amino-3-phenyl-1-propanol
and
6.91 g (50 mmol) of potassium carbonate in 50 mL of 96%
ethanol
and 10 mL of water is brought to reflux temperature. To this stirred, two-phase mixture is added dropwise
10.69 g (62.5 mmol) of benzyl bromide
. The vigorously stirred mixture is heated at reflux for an additional 0.5 hr and cooled to room temperature, and 30 mL of water are added. The product is extracted three times with
100 mL of ether
, and the combined organic phases are washed with a saturated
NaCl solution and dried over
MgSO4
. Following removal of the solvents using a
rotary evaporator, the crude product is recrystallized from
20 mL of boiling hexane
to provide
7.92 g (
96% yield) of white crystals having a melting point of
68-69°C. The
1H and
13C NMR spectra and enantiomeric purity are identical to those previously recorded
(Note 5).
7.
The oxidation can also be performed efficiently with
pyridine/sulfur trioxide
, but not with Jones reagent;
pyridinium dichromate
affords poor yields (40-50%).
4
8.
Thin layer chromatography of the crude product shows one spot with R
f = 0.64. The material can be purified by column chromatography (
silica gel;
petroleum ether/ethyl acetate 95:5, v/v) to provide a
92% yield of analytically pure aldehyde.
4 However, in further reactions of the aldehyde it is best to use the crude material as it is. The spectral properties are as follows:
1H NMR (400 MHz, CDCl
3) δ: 2.94 and 3.15 (ABX System, 2 H, J
AB = 13.9, J
AX = 7.3 and J
BX = 6.2, C
6H
5-CH
2), 3.56 (t, 1 H, 7.1, CH
2-CH-N), 3.69 and 3.82 (AB System, 4 H, J
AB = 13.7, N-CH
2), 7.12-7.30 (m, 15 H, N-CH
2-C
6
H
5 and C
6
H
5-CH
2) and 9.72 (s, 1 H, C-CHO)
;
13C NMR (100 MHz, CDCl
3) δ: 29.2 (t, C
6H
5-CH
2), 53.8 (t, N-CH
2), 67.5 (d, CH
2-CH-N), 125.2-138.1 (M, N-CH
2-C
6
H
5 and C
6
H
5-CH
2) and 200.9 (d, C-CHO)
. Anal. Calcd for C
23H
23NO: C, 83.85; H, 7.04; N, 4.25. Found: C, 84.02; H, 7.13; N, 4.23.
9.
The procedure may be conducted on a larger scale. For example, the submitters used
40 mmol of (S)-2-(N,N-dibenzylamino)-3-phenyl-1-propanol
, and obtained
12.9 g (
98%) of the aldehyde.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure described here provides a simple way to prepare S-configured
2-(N,N-dibenzylamino)-3-phenylpropanal from naturally occurring
phenylalanine
using protocol that is quite general for the conversion of naturally occurring α-amino acids into the corresponding N,N-dibenzyl-protected α-amino aldehydes.
5,6 The amino acids that have been used so far include
alanine
,
5,6
phenylalanine,
5,6
valine
,
5,6 leucine,
5,6
isoleucine
,
6
lysine
,
6
serine
,
6
threonine
,
6
ornithine
,
6 and
tryptophan
.
7 Upon using the enantiomers of the natural α-amino acids, R-configured N,N-dibenzyl-protected α-amino aldehydes may be prepared.
6,8
N,N-Dibenzylamino aldehydes are useful building blocks in a wide variety of diastereoselective C-C bond forming reactions. These include: Grignard-type reactions
5,6,9 of RMgX, RLi, R
2CuLi, or RTi(OiPr)
3; aldol additions involving lithium (Li) enolates,
5,6,8
zinc
reagents,
10 enolsilanes,
6,8,11 or enolboranes;
12
trimethylsilyl cyanide (Me3SiCN) additions catalyzed by ZnX
2;
6,13 sulfur ylide additions;
6,8,14 and hetero Diels-Alder reactions
6,8 (Scheme 1). In almost all cases a high degree of non-chelation control (diastereoselectivity 90-99%) has been observed, an unusual phenomenon that can be explained either by applying the Felkin-Anh model or by invoking ground state effects.
6,15 If highly Lewis acidic conditions are used, reversal of diastereoselectivity may result, but chelation control is not general.
5,6
A number of research groups have used N,N-dibenzylamino aldehydes in these and in other C-C bond forming reactions.
6,7,8,9,10,11,12,13,14,16 In most cases the products were shown to be enantiomerically pure (ee > 98%), which demonstrates the absence of any appreciable racemization during the formation or reaction of these aldehydes. Nevertheless, it is best to use freshly prepared samples in crude form as soon as possible. During isolation of the N,N-dibenzylamino aldehydes it is not necessary to work at low temperatures. This is in contrast to the configurationally much more labile N-tert-butoxycarbonyl(Boc)-protected analogs, that have to be handled in cold
ether.
6,17 The N-Boc-protected α-amino aldehydes generally react more or less stereo-randomly with such reagents as RLi, RMgX, Li-enolates, NaCN/NaHSO
3, or sulfur ylides,
6,17 the so-called Garner aldehyde derived from
serine being a notable exception.
6,18 Under special Lewis acidic conditions N-Boc-protected α-amino aldehydes afford the chelation controlled products,
17,18,19 which means that such processes are stereochemically complementary to the reactions of N,N-dibenzylamino aldehydes. Deprotection of the reaction products, i.e., removal of the two benzyl groups at
nitrogen, is best achieved using the Pearlman catalyst according to the method of Yoshida.
6,20
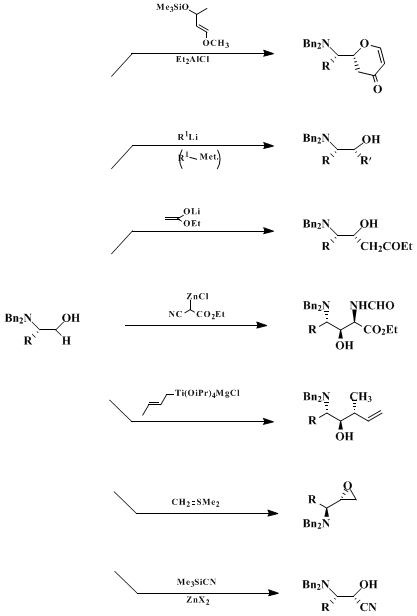
Scheme 1
Typical Non-chelation-Controlled Reactions of N,N-Dibenzylamino Aldehydes
N,N-Dibenzylamino aldehydes are also useful building blocks in the preparation of other classes of amino compounds as summarized in a review article covering the literature up to mid 1998.
6 These include αβ-unsaturated ester or ketone derivatives prepared by Wittig or Wittig-Horner reactions
21 as well as α-amino aldimines prepared by condensation reactions of the aldehydes.
22 Such compounds are in themselves synthetically interesting building blocks in a variety of C-C bond forming processes, Michael additions and hydrogenation reactions.
21,22 The industrial synthesis and use of the title compound has been described on a 190 kg (576 mol) scale.
23 Finally, N,N-dibenzylamino aldehydes and ketones have been prepared in enantiomerically pure form from precursors other than α-amino acids, adding to the synthetic versatility of this class of compounds.
24
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
S-2-(N,N-Dibenzylamino)-3-phenylpropanal:
Benzenepropanal, α-[bis(phenylmethyl)amino]-, (S)- (12); (111060-64-1)
Lithium aluminum hydride:
Aluminate (1-), tetrahydro-, lithium (8);
Aluminate (1-) tetrahydro-, lithium, (I-4)- (9); (16853-85-3)
Benzyl (S)-2-(N,N-dibenzylamino)-3-phenylpropanoate:
L-Phenylalanine, N,N-bis(phenylmethyl)-, phenylmethyl ester (12); (111138-83-1)
(S)-Phenylalanine:
Alanine, phenyl-, L- (8);
L -Phenylalanine (9); (63-91-2)
Benzyl bromide:
Toluene, α-bromo- (8);
Benzene, (bromomethyl)- (9); (100-39-0)
(S)-2-(N,N-Dibenzylamino)-3-phenyl-1-propanol:
Benzenepropanol, β-[bis(phenylmethyl)amino]-, (S)- (12); (111060-52-7)
Benzyl alcohol (8);
Benzenemethanol (9); (100-51-6)
Oxalyl chloride (8);
Ethanedioyl dichloride (9); (79-37-8)
Dimethyl sulfoxide:
Methyl sulfoxide (8);
Methane, sulfinylbis- (9); (67-68-5)
Triethylamine (8);
Ethanamine, N,N-diethyl- (9); (121-44-8)
(S)-2-Amino-3-phenyl-1-propanol:
1-Propanol, 2-amino-3-phenyl-, L- (8);
Benzenepropanol, 2-amino-, (S)- (9); (3182-95-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved