Org. Synth. 2000, 77, 50
DOI: 10.15227/orgsyn.077.0050
SULFINIMINES (THIOOXIMINE S-OXIDES): ASYMMETRIC SYNTHESIS OF METHYL (R)-(+)-β-PHENYLALANATE FROM (S)-(+)-N-(BENZYLIDENE)-p-TOLUENESULFINAMIDE
[
Benzenepropanoic acid, β-amino-, (R)-, methyl ester from Benzenesulfinamide, 4-methyl-N-(phenylmethylene)- [S-(E)]-
]
Submitted by Dean L. Fanelli, Joanna M. Szewczyk, Yulian Zhang, G. Venkat Reddy, David M. Burns, and Franklin A. Davis
1
.
Checked by James Unch and David J. Hart.
1. Procedure
A. (S)-(+)-p-Toluenesulfinamide
(2)
:
2 An oven-dried,
1-L, one-necked, round-bottomed flask
(Note 1), equipped with a
magnetic stirring bar and
rubber septum is charged with
30.31 g (0.103 mol) of (1R,2S,5R)-(−)-menthyl (S)-p-toluenesulfinate
(1)
(Note 2) under an
argon purge. The reaction flask is placed under vacuum for 1 hr to remove residual moisture,
220 mL of anhydrous tetrahydrofuran
(Note 3) is added via cannula, and the solution is rapidly stirred and cooled to −78°C using a
dry ice/acetone bath. To the white suspension
(Note 4) is added
143.0 mL (0.143 mol) of 1.0 M lithium bis(trimethylsilyl)amide (LiHMDS)
(Note 5) via syringe. The dry ice/acetone bath is removed and the solution is stirred for 4.5 hr at room temperature. The mixture is then cooled to −78°C in a dry ice/acetone bath,
30% aq ammonium chloride (120 mL) is added, and the mixture is warmed to room temperature. The solution is transferred to a
1-L separatory funnel, diluted with water (80 mL), and extracted with
diethyl ether (3 × 160 mL). The combined organic phases are washed with water (2 × 120 mL),
brine (120 mL), dried over anhydrous
sodium sulfate
, filtered into a 1-L, single-necked, round-bottomed flask, and concentrated using a
rotary evaporator
(Note 6). To the residual yellow paste is added
pentane (100 mL). After standing overnight in the refrigerator (3°C), and warming to room temperature, the resulting solid is collected by suction filtration. The filter cake is rinsed with
pentane (2 × 50 mL) and dried under vacuum to afford
11.52 g (
72%) of analytically pure
2 as a white solid
(Note 7).
B. (S)-(+)-N-(Benzylidene)-p-toluenesulfinamide
(3)
:
2 An oven-dried,
500-mL, one-necked, round-bottomed flask
(Note 1), equipped with a magnetic stirring bar and rubber septum is charged with
10.35 g (66.8 mmol) of (S)-(+)-p-toluenesulfinamide
(2)
. The reaction flask is placed under vacuum (1 mm) for 30 min to remove residual moisture. The vessel is then placed under an
argon atmosphere and
dichloromethane (135 mL)
(Note 8) is added via cannula. The solution is stirred rapidly and
7.07 g (66.6 mmol) of benzaldehyde
(Note 9) is added via syringe, followed by
76.0 g (333 mmol) of titanium(IV) ethoxide
(Note 8) via cannula. The septum is removed, the solution is fitted with a reflux condenser topped with an
argon gas line and the yellow solution is heated under reflux for 5 hr. The solution is cooled to room temperature and poured into water (85 mL) in a
600-mL beaker with rapid stirring
(Note 10).
Dichloromethane (50 mL) is added to the mixture, which is then filtered through a
Buchner funnel using filter paper of medium porosity. The filter cake is rinsed with
dichloromethane (3 × 70 mL). The filtrate is transferred to a
500-mL separatory funnel and washed with water (3 × 70 mL),
brine (70 mL), dried over
magnesium sulfate (MgSO4) and filtered. The solution is concentrated using a rotary evaporator while keeping the bath temperature below 40°C to afford
15.0 g (
92%) of analytically pure product as a white solid
(Note 11).
C. (Ss,R)-(+)-Methyl N-(p-toluenesulfinyl)-3-amino-3-phenylpropanoate
(4):
3 An oven-dried,
250-mL, round-bottomed flask equipped with a
magnetic stirrer and a rubber septum is charged with
8.4 mL of sodium bis(trimethylsilyl)amide (NaHMDS) (16.7 mmol) in tetrahydrofuran
(Note 12). The reaction mixture is diluted with
80 mL of anhydrous ether
(Note 13), the solution is cooled to −78°C using a dry ice/acetone bath, and
1.3 mL of methyl acetate (16.7 mmol)
(Note 14) is added dropwise via syringe over 20 min. In a separate, oven-dried,
100-mL, round-bottomed flask, equipped with a magnetic stirrer, rubber septum and under an
argon purge, is placed
2.65 g (10.9 mmol) of (S)-(+)-N-(benzylidene)-p-toluenesulfinamide
(3) in 50 mL of anhydrous ether
. The solution of
3 is cooled to 0°C and added by cannula over 60 min into the reaction flask containing the enolate. The flask is rinsed with
10 mL of anhydrous ether
and cooled to −78°C prior to adding it to the reaction mixture by cannula. After the mixture is stirred for 45 min, it is quenched at this temperature with
4 mL of aqueous saturated ammonium chloride
(Note 15), warmed to room temperature, and diluted with
50 mL of ethyl acetate
. The solution is transferred to a
250-mL separatory funnel, washed with water (2 × 50 mL), and the aqueous phases are combined and washed with
ethyl acetate (2 × 20 mL). The combined organic phases are washed with aqueous saturated
sodium chloride (2 × 40 mL), dried over anhydrous
magnesium sulfate and filtered. The solution is concentrated using a rotary evaporator (33°C and 13 mm), followed by drying under high vacuum (30°C and 0.05 mm) to give a yellow solid (
2.7 g) (mp
82-85°C). This material is dissolved in
5 mL of ethyl acetate
and
5 mL of methylene chloride
, diluted with
125 mL of pentane
, and stored at −20°C overnight
(Note 16). The slightly yellow suspended solid is filtered, washed with cold
pentanes (2 × 15 mL; ca. −20°C), and dried under high vacuum to remove residual solvent to afford
2.1-2.3 g (
61-66%) of analytically pure
4
(Note 17),
(Note 18) and
(Note 19).
D. Hydrolysis of (Ss,R)-(+) Methyl N-(p-tolylsulfinyl)-3-amino-3-phenylpropanoate
(4): An oven-dried, 250-mL, round-bottomed flask equipped with a magnetic stirring bar is charged with
2.13 g (6.7 mmol) of (Ss,R)-(+)-methyl N-(p-tolylsulfinyl)-3-amino-3-phenylpropanoate
(4)
, sealed with a rubber septum, and
100 mL of methanol
is added (Note 20). Stirring is initiated and the white mixture is cooled to 0°C, in an ice/water bath, prior to adding
1.9 mL (24.6 mmol) of trifluoroacetic acid
(Note 21) via cannula over 3 min. After 10 min the solution is warmed to room temperature and stirred for 2-4 hr (Note 22). The solvent is removed using a rotary evaporator. The resulting yellow liquid is diluted with
15 mL of diethyl ether
(Note 23) and transferred to a 250-mL separatory funnel. The flask is rinsed with
diethyl ether (2 × 30 mL) and the combined organic phases are extracted with
15% aqueous hydrochloric acid (HCl) (2 × 75 mL)
(Note 24). The combined aqueous HCl extracts are washed with
diethyl ether (50 mL)
(Note 25) and then transferred to a 400-mL beaker containing
dichloromethane (50 mL) and a magnetic stirring bar. The solution is cooled to 0°C using an ice/water bath, and solid
sodium bicarbonate (NaHCO3
) is carefully added to adjust the pH to 8 (Note 26) and (Note 27). The resulting emulsion is transferred to a 250-mL separatory funnel, diluted with
25 mL of methylene chloride
, and the aqueous layer is extracted with
methylene chloride (3 × 50 mL). The organic phases are washed with 50 mL of water, saturated brine (2 × 50 mL) and dried over anhydrous magnesium sulfate. Removal of the solvent on a rotary evaporator affords 0.99 g of crude
methyl (R)-(+)-β-phenylalanate
as a viscous yellow oil (Note 28). This material is purified by bulb-to-bulb distillation to give 0.813 g (68%) of 5 as a water white liquid (Note 29).
2. Notes
1.
All glassware was predried at 120°C for at least 4 hr and cooled to room temperature prior to use in a desiccator.
2.
(1R,2S,5R)-(−)-Menthyl (S)-p-toluenesulfinate was purchased from Aldrich Chemical Company, Inc.
, or can be prepared by the following procedures: (a) Hulce, M.; Mallamo, J. P.; Frye, L. L.; Kogan, T. P.; Posner, G. H.
Org. Synth., Coll. Vol. VII
1990, 495; (b) See also Reference
4
3.
Reagent grade
tetrahydrofuran
was freshly distilled under
nitrogen from a purple solution of
sodium and benzophenone
.
4.
Upon cooling to −78°C, the solution of
(1R,2S,5R)-(−)-menthyl (S)-p-toluenesulfinate
(1) forms a white milky precipitate.
5.
Lithium bis(trimethylsilyl)amide in tetrahydrofuran (1.0 M) solution was purchased from Aldrich Chemical Company, Inc.
6.
The submitters indicate that use of a
high vacuum pump results in removal of most of the
menthol at this point. The checkers used a standard rotary evaporator (33°C at 16 mm) and observed that the residual yellow paste retains the
menthol.
7.
The physical properties of
(S)-(+)-p-toluenesulfinamide were as follows: mp
110-112°C;
[α]
D
20 +79.7° (CHCl
3,
c1.2), IR (KBr) cm
−1: 3200, 3094
;
1H NMR (CDCl
3) δ: 2.42 (s, 3 H), 4.33 (s, 2H), 7.32 (d, 2 H, J = 8), 7.64 (d, 2 H, J = 8)
;
13C NMR (CDCl
3) δ: 21.3, 125.3, 129.5, 141.4, 143.4
. Anal. Calcd for C
7H
9NOS: C, 54.18; H, 5.85. Found: C, 54.22; H, 5.86.
8.
Anhydrous
methylene chloride and titanium(IV) ethoxide (technical grade) were purchased from Aldrich Chemical Company, Inc.
, and used as received.
9.
Benzaldehyde was used from a freshly opened bottle purchased from Aldrich Chemical Company, Inc.
10.
A magnetic stirring bar is used for initial stirring. A thick slurry develops and when magnetic stirring becomes impossible, the mixture is stirred with a metal spatula for about 1 min.
11.
The physical properties of
(S)-(+)-N-(benzylidene)-p-toluenesulfinamide were as follows: mp
80-81°C ee >95%;
[α]
D
20 +122.8° (
CHCl3
,
c 1.2); IR (KBr) cm
−1: 3050, 1607, 1574, 1449, 1104, 1072
;
1H NMR (CDCl
3) δ: 2.4 (s, 3 H), 7.32 (d, 2 H, J = 8.0), 7.4-7.52 (m, 3 H), 7.64 (d, 2 H, J = 8.0), 7.81-7.86 (m, 2 H), 8.74 (s, 1 H)
;
13C NMR (CDCl
3) δ: 21.5, 124.7, 128.8, 129.5, 129.7, 132.5, 133.7, 141.6, 141.7, 160.0
. Anal. Calcd for C
14H
13NOS: C, 69.11; H, 5.39. Found: C, 68.84; H, 5.50.
12.
Sodium bis(trimethylsilyl)amide in tetrahydrofuran (2.0 M) was purchased from Acros Chemical Company, Inc.
13.
Reagent grade anhydrous ether
was freshly distilled under
argon from a purple solution of
sodium and
benzophenone.
14.
Anhydrous
methyl acetate was purchased from Aldrich Chemical Company, Inc.
, and used without further purification.
15.
The submitters indicate that completion of the reaction is confirmed by TLC on
silica gel
using
50%
ethyl acetate in hexanes
as the eluant.
16.
The submitters indicate that
4 can also be purified by flash chromatography using
25%
ethyl acetate/hexanes on silica gel (30 g/g of crude product), Merck
grade 60 (230-400 mesh) was purchased from Aldrich Chemical Company, Inc.
17.
The submitters indicate that the observed yield was
89% when the reaction was carried out on a 1.0-g scale.
18.
The diastereomeric excess was determined by
1H NMR (300 MHz, CDCl
3) by evaluating the p-tolyl methyl group (major δ 2.41 ppm: minor δ 2.36 ppm) or carbomethoxy group (major δ 3.60 ppm: minor δ 3.64 ppm).
19.
The spectral properties of
(Ss,R)-(+)-methyl N-(p-tolylsulfinyl)-3-amino-3-phenylpropanoate
(4) are as follows: >98% de;
[α]
D
20 116.84° (
CHCl3
,
c. 1.74) [checkers recorded
[α]
D
18 111.1° (CHCl
3,
c 1.74)]; mp
88-89°C; IR (KBr) cm
−1: 3155, 1737, 1436, 1295, 1170, 1044, 804, 700
;
1H NMR (CDCl
3) δ: 2.41 (s, 3 H), 2.86 (d, 2 H, J = 6.3), 3.60 (s, 3 H), 4.90 (q, 1 H, J = 6.1), 5.01 (d, 1 H, J = 5.4), 7.28-7.45 (m, 7 H), 7.60 (d, 2 H, J = 8.2)
;
13C NMR (CDCl
3) δ: 21.2, 41.9, 51.7, 54.7, 125.3, 127.1, 127.9, 128.6, 129.4, 140.3, 141.3, 142.1, 171.1
; MS m/z 317 (M
+), 269, 196, 178, 139, 121, 104, 91, 77
. Anal. Calcd for C
17H
19NO
3S: C, 64.33; H, 6.03. Found: C, 64.38; H, 6.12.
20.
A freshly opened bottle of
methanol (certified A.C.S., purchased from Aldrich Chemical Company, Inc.) was used without further purification or drying.
21.
Trifluoroacetic acid
99% was purchased from Aldrich Chemical Company, Inc.
22.
The submitters indicate that the reaction can be monitored for the formation of
5 by thin layer chromatography (
silica gel;
50%
EtOAc/hexanes
).
23.
Reagent grade anhydrous ethyl ether was purchased from Aldrich Chemical Company, Inc.
24.
Aqueous
hydrochloric acid (37%) certified A.C.S. PLUS was purchased from Fisher Chemical Fisher Scientific
and diluted to 15% with water.
25.
The checkers find that this wash eliminates
methyl p-toluenesulfinate
as a contaminant in the crude product.
26.
Certified
A.C.S. grade
methylene chloride was purchased from Aldrich Chemical Company, Inc.
and used as received.
Sodium bicarbonate
A.C.S. grade was purchased from Fisher Scientific Company
.
27.
The final pH should be at least 8.0 (by pH paper) to ensure that all the salt has been deprotonated.
28.
The checkers recorded
[α]
D
18 +18.9° (CHCl
3,
c 1.85) for this material and noted some unidentified impurities in the
1H NMR spectrum. The submitters indicate that
3 mL of methanol
can be added to the yellow liquid followed by filtration to remove precipitated solids.
29.
The spectral properties of this material are as follows: bp
55-60°C (oven temperature) at 0.05 mm; >98 % ee,
[α]
D
20 +22.6° (CHCl
3,
c 1.85); IR (neat) cm
−1: 3378, 3026, 2950, 1734, 1603, 1436, 1171, 1020, 762, 700
;
1H NMR (CDCl
3) δ: 1.87 (br s, 2 H, exchangeable with D
2O), 2.66 (d, 2 H, J = 6.9), 3.68 (s, 3 H), 4.42 (t, 1 H, J = 6.7), 7.25-7.35 (m, 5 H)
;
13C NMR (CDCl
3) δ: 43.9, 51.5, 52.5, 126.1, 127.3, 128.5, 144.6, 172.4
. Anal. Calcd for C
10H
13NO
2: C, 67.00; H, 7.32. Found: C, 66.50; H, 7.41.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
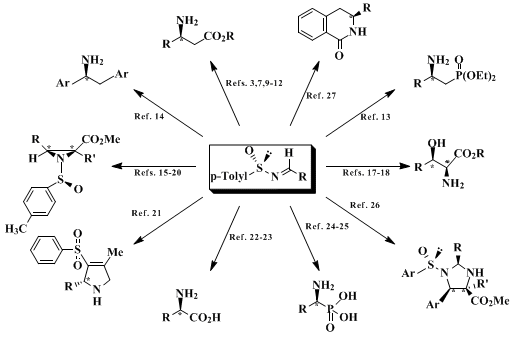
Although the diastereoselective addition of nucleophiles to imines offers an attractive route to chiral amine derivatives, most chiral nonracemic imines suffer from low reactivity (electrophilicity), resulting in no reaction or competitive reduction with organometallic reagents. Other problems include enolization of aliphatic imines, poor diastereoselectivities caused by syn/anti isomerism, and moisture sensitivity resulting in moderate or low yields. When primary amines are the objective, removing the N-auxiliary often leads to epimerization or destruction of the product.
The N-sulfinyl auxiliary in sulfinimines [ArS(O)-N=CHR, thiooxime S-oxides] offers a general solution for the control of imine reactivity and diastereoselectivity.
5 In sulfinimines the electron-withdrawing N-sulfinyl group activates the C-N bond to such an extent that enolization of aliphatic examples is no longer a significant problem. Furthermore, the sulfinyl auxiliary exerts powerful stereodirecting effects in the addition of enolates and other organometallic reagents. Following separation of the diastereomeric sulfinamide [ArS(O)NH-CHRR'], hydrolysis affords the primary amine derivative without epimerization. Moreover, the sulfinamide N-sulfinyl group can be used for further elaboration of the product.
An earlier synthesis of sulfinimines, involving the addition of metal ketimines to the
menthyl p-toluenesulfinate
(Andersen reagent), was limited because an aromatic group was required to be present and the more valuable aldehyde-derived sulfinimines were unavailable.
6 An asymmetric oxidation approach using chiral oxaziridines suffered from moderate ee's (<90%).
7 tert-Butylsulfinimines are available in a series of steps starting with
tert-butyl disulfide
.
8 The method described here affords these valuable building blocks from commercially available starting materials and aromatic and aliphatic aldehydes.
2 Although β-amino acids are less common than α-amino acids, they are important constituents of natural products, precursors of the β-lactams and increasingly used to modify proteins.
9 The synthesis of
β-phenylalanine methyl ester
is an example of the general synthesis of this important class of amino acids using sulfinimines.
3,6,10,11,12 Exclusive formation of the sulfinamide
4 is probably a consequence of the anion stabilizing N-sulfinyl group; analogous reactions of N-alkyl- and N-arylimines produce cyclized β-lactams. An added feature of the N-sulfinyl group in
4 is that it is easily removed under mild conditions.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Methyl (R)-(+)-β-phenylalanate:
Benzenepropanoic acid, β-amino-, (R)-, methyl ester
(9); (37088-67-8)
(S)-(+)-N-(Benzylidene)-p-toluenesulfinamide:
Benzenesulfinamide, 4-methyl-N-(phenylmethylene)-, [S-(E)]- (13); (153277-49-7)
(S)-(+)-p-Toluenesulfinamide:
Benzenesulfinamide, 4-methyl-, (S)- (14);
(188447-91-8)
(1R,2S,5R)-(−)-Menthyl (S)-p-toluenesulfinate:
Menthol, (−)-, (S)-p-toluenesulfinate (8);
Benzenesulfinic acid, 4-methyl-, 5-methyl-2-(1-methylethyl)cyclohexyl ester,
[1R-[1α(S),2β,5α]]- (9); (1517-82-4)
Lithium bis(trimethylsilyl)amide:
Disilazane, 1,1,1,3,3,3-hexamethyl-, lithium salt (8);
Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, lithium salt (9); (4039-32-1)
Benzaldehyde (8,9); (100-52-7)
Titanium(IV) ethoxide:
Ethyl alcohol, titanium(4+) salt (8);
Ethanol, titanium(4+) salt (9);
(3087-36-3)
(SsR)-(+)-Methyl N-(p-toluenesulfinyl)-3-amino-3-phenylpropanoate:
Benzenepropanoic acid, β-[[(4-methylphenyl)sulfinyl]amino]-, methyl ester, [S-(R,S)]-
(13); (158009-86-0)
Sodium bis(trimethylsilyl)amide:
Disilazane, 1,1,1,3,3,3-hexamethyl-, sodium salt (8);
Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, sodium salt (9); (1070-89-9)
Methyl acetate:
Acetic acid, methyl ester (8,9); (79-20-9)
Trifluoroacetic acid:
Acetic acid, trifluoro- (8,9); (76-05-1)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved