Checked by Robert E. Lee Trout and Amos B. Smith, III.
1. Procedure
2. Notes
1.
It is most convenient to attach cooling water in series to the free condenser first and then to the cooling jacket on the complexation flask.
2.
The submitters used
nitrogen at this point, but the checkers found that
argon worked as well. The checkers also recommend the use of an
Oxiclear gas purifier.
3.
Fresh reagent grade
acetonitrile was purchased from Fisher Scientific Co.
and used without additional purification.
Chromium hexacarbonyl was purchased from Strem Chemical Co.
Celite and
cycloheptatriene (90% technical grade) were purchased from Aldrich Chemical Company, Inc.
, and used without purification. THF was distilled from
sodium/benzophenone ketyl
.
4.
Once heating of the reaction is begun, any significant cooling or exposure to the atmosphere generally causes degradation of the
tris(acetonitrile)chromium tricarbonyl intermediate. The reaction initially turns greenish yellow, but then quickly forms a bright yellow to golden color that becomes dark green upon degradation. Greenish, partially degraded intermediates can be carried through the sequence with a corresponding reduction in yield. The total time of reflux ranged from 24-26 hr.
5.
This item may be purchased from Ace Glass Inc., Vineland, N.J., catalog #8766-12.
6.
Vacuum must be applied carefully to avoid bumping, but must also be applied quickly and steadily to avoid degradation of the reaction intermediate.
7.
Warning!
Tris(acetonitrile)chromium tricarbonyl is highly pyrophoric and degrades rapidly when exposed to
oxygen, but is reasonably stable in THF solution. Best yields are obtained when this intermediate is as free of
acetonitrile as possible while avoiding formation of the green colored [Cr(III)] decomposition product, which develops on contact with air.
8.
The reaction is monitored by TLC (
silica gel, 6:1 hexanes:
ethyl acetate). Typical characteristics are R
f = 0.15, a yellow spot [
tris(acetonitrile)chromium tricarbonyl intermediate], and R
f = 0.51, a red spot (product complex). Total reaction time averaged ~180 hr.
9.
Solvent is removed via rotary evaporator.
10.
This product was typically found to be ≥ 98% pure based on
1H NMR analysis, and it may be used without further purification. However, the compound may be recrystallized from hexanes if necessary. The complex exhibits the following characteristics: TLC: R
f = 0.51 (
silica gel, 6:1
hexanes:
ethyl acetate);
1H NMR (500 MHz, CD
2Cl
2) δ: 1.74 (d, 1 H), 2.95 (dt, 1 H, J = 9.0, 14.0), 3.40 (t, 2 H, J = 7.5), 4.87 (bs, 2 H), 6.09 (bs, 2 H)
;
13C NMR (125 MHz, CD
2Cl
2) δ: 23.9 (CH
2), 57.1 (CH), 98.4 (CH), 101.1 (CH)
; IR (CDCl
3) cm
−1: 3052, 2895, 2848, 1982, 1974, 1917, 1897, 1886, 1877
; HRMS calcd for C
10H
8CrO
3: m/e 227.9879, found 227.9881
; LRMS [EI] (rel. %): 227.9 (19), 199.9 (13), 172.0 (15), 144.0 (74)
.
11.
Performing this reaction at higher concentrations (i.e., in 1-2 L solvent) results in significantly increased reaction times, incomplete reaction, and increased side product formation.
12.
The reaction conditions given were developed using
(E)-1-acetoxy-1,3-butadiene
prepared according to the procedure of McDonald, et al.
2 with the following modifications (unchecked).
Crotonaldehyde (105 g, 125 mL) is added by addition funnel over 1 hr to a refluxing solution of
isopropenyl acetate (2.5 mol, 250 g, 275 mL),
p-toluenesulfonic acid (anhydrous, 2.0 g) and
copper(II) acetate (0.5 g). The mixture is heated at reflux for ~30 min and then the reaction apparatus is set up for distillation. Distillation (bath temp. 110-130°C) is continued for ~2.5 hr until
acetone and nearly all unreacted
isopropenyl acetate is collected. The distillation residue is cooled to ~25°C and crude product is isolated via vacuum distillation (bp ~32°C, ~7 mm). This crude product typically contains traces of
isopropenyl acetate and significant amounts of
acetic acid
. The crude distillate is dissolved in
diethyl ether (500 mL), and carefully mixed with
saturated aqueous sodium bicarbonate
solution, adding additional anhydrous
sodium bicarbonate slowly to the stirring mixture until gas evolution ceases and the pH increases to ~7.0. The layers are separated and the organic phase is washed with
brine (300 mL) and dried with
magnesium sulfate
. The solution is carefully concentrated, and the product is purified by distillation to yield nearly pure
(E)-1-acetoxy-1,3-butadiene (
~35-50% yield). Frequently, sequential distillations of the product are necessary to ensure the purity of the product obtained. Pure product exhibits the following characteristics: bp
32°/10 mm; TLC: R
f = 0.61 (
silica gel, 6:1
hexanes:
ethyl acetate);
1H NMR (500 MHz, CDCl
3) δ: 2.14 (s, 3 H), 5.08 (dd, 1 H, J = 10.5, 0.5), 5.21 (d, 1 H, J = 17.0), 6.03 (dd, 1 H, J = 12.0, 12.0), 6.26 (ddd, 1 H, J = 21.5, 10.5, 10.5), 7.39 (d, 1 H, J = 12.5)
;
13C NMR (125 MHz, CDCl
3) δ: 20.7 (CH
3), 116.0 (CH), 117.3 (CH
2), 131.7 (CH), 138.6 (CH), 167.8 (C)
; IR (CDCl
3) cm
−1: 3091, 3074, 3041, 1660, 1097
; HRMS m/e calcd for C
6H
8O
2: 112.0524, found 112.0523
; LRMS [EI] (rel %): 112.0 (57), 70.0 (100)
.
Alternatively,
1-acetoxy-1,3-butadiene is available as a mixture of E,Z-isomers from Aldrich Chemical Company, Inc.
When using the commercial reagent, 3.0 eq. (14.8 g, 15.6 mL) is necessary to ensure complete reaction, as the Z isomer does not react.
13.
Caution: UV radiation is harmful to eyes and skin; the reaction vessel may be wrapped with aluminum foil or the reaction conducted in a closed photochemical reaction cabinet to prevent exposure to the harmful UV rays.
14.
The photochemical lamp and power supply may be purchased from Ace Glass Inc., Vineland, N.J., catalog #'s 7825-32 or 7825-40 (lamp) and 7830-60 (power supply).
15.
A solid buildup occurs on the immersion well that may slow the reaction considerably. To help minimize this, the submitters suggest a constant purging of the reaction mixture with
argon throughout the entire reaction time.
16.
Typical TLC data (
silica gel, 6:1
hexanes:
ethyl acetate) include R
f = 0.61 (
1-acetoxy-1,3-butadiene); 0.51, a red spot [
tricarbonyl(cycloheptatriene)chromium]; 0.45 a yellow spot (side product that often overlaps with the starting complex); and 0.31 a yellow spot (main intermediate chromium complex).
17.
Prior to and between washes, the green filter cake cracks and should be "pushed down" with a spatula to form a uniform surface prior to any subsequent washes.
18.
TLC at this point (
silica gel, 6:1 hexanes:
ethyl acetate) shows three spots (UV): R
f = 0.76 (trace orange); 0.55 (side product); 0.47 (main product).
19.
Chromatography is performed as follows: a
3.5-cm ID glass column is packed with
~140 g of flash grade silica gel (Merck 230-400 mesh) in
petroleum ether
and the sample is loaded in minimal
petroleum ether. The checkers found that a
5.0-cm ID glass column packed with ~170 g of Merck 70-270 mesh silica gel gave slightly better separation. Care must be taken during product application to minimize silica gel column separation. The column is eluted, recycling solvent as necessary, until the front running orange band is collected. This band is comprised of trace amounts of unreacted
tricarbonyl(cycloheptatriene)chromium. Elution then proceeds using
500 mL of 49:1 petroleum ether:diethyl ether
followed by 19:1
petroleum ether:
diethyl ether to obtain the product. Prior to elution of the desired [6π+4π] cycloadduct, the side product, [6π+2π] cycloadduct
(A) elutes, usually streaking into the desired product, but it is of little consequence. All fractions containing the desired product are combined and the solvent is removed under reduced pressure. The product sometimes solidifies during solvent removal, but may require seeding with authentic material to promote crystallization.
20.
The [6π+4π] cycloadduct exhibits the following characteristics: bp:
104-107°/1.3 mm; TLC: R
f = 0.47 (
silica gel, 6:1
hexanes:
ethyl acetate);
1H NMR (500 MHz, CDCl
3) δ: 2.11 (s, 3 H), 2.12-2.15 (m, 1 H), 2.31 (bd, 1 H, J = 14.0), 2.35-2.47 (m, 2 H), 2.74 (bs, 1 H), 2.92 (bs, 1 H), 5.49 (bd, 1 H, J = 11.0), 5.60-5.65 (m, 1 H), 5.66-5.68 (m, 1 H), 5.73-5.81 (m, 2 H), 5.83-5.88 (m, 2 H)
;
13C NMR (125 MHz, CDCl
3) δ: 21.4 (CH
3), 31.7 (CH
2), 32.9 (CH
2), 37.3 (CH), 42.7 (CH), 76.7 (CH), 124.9 (CH), 127.1 (CH), 128.7 (CH), 133.1 (CH), 135.3 (CH), 137.8 (CH), 170.5 (C)
; IR (neat) cm
−1: 3011, 2924, 2905, 2884, 2872, 1737, 1447, 1430, 1368, 1241, 1199, 1055, 1020
; HRMS calcd for C
13H
16O
2: m/e 204.11503, found 204.1149
; LRMS [EI] (rel %): 204.1 (2), 162.1 (2), 144.1 (20), 129.0 (11), 112.0 (6), 92.0 (100)
. Purity was determined by 500 MHz
1H NMR, with the main impurity being the
[6π+2π] cycloadduct
A.
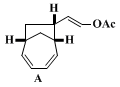
This compound exhibits the following characteristics: TLC: Rf = 0.35 (silica gel, 19:1 hexanes:ethyl acetate);
1H NMR (500 MHz, CDCl3) δ: 1.58 (ddd, 1 H, J = 13.5, 9.5, 3.5), 1.89 (d, 1 H, J = 12.0), 2.01 (ddd, 1 H, J = 13.5, 9.5, 9.5), 2.10 (s, 3 H), 2.14-2.19 (m, 1 H), 2.61 (dd, 1 H, J = 12.0, 5.5), 2.69 (ddd, 1 H, J = 16.5, 8.5, 4.0), 2.84 (ddd, 1 H, J = 19.5, 9.5, 6.0), 5.58 (d, 1 H, J = 10.0, 6.0), 5.62 (dd, 1 H, J = 12.0, 9.5), 5.72 (dd, 1 H, J = 12.0, 7.0), 5.83 (dd, 1 H, J = 12.0, 6.5), 6.10 (dd, 1 H, J = 10.5, 8.5), 7.09 (d, 1 H, J = 12.0)
;
13C NMR (125 MHz, CDCl3) δ: 20.7 (CH3), 33.3 (CH2), 36.7 (CH), 42.3 (CH2), 46.3 (CH), 54.7 (CH), 115.5 (CH), 123.3 (CH), 126.6 (CH), 135.0 (CH), 135.2 (CH), 141.0 (CH), 168.2 (C)
; IR (neat) cm−1: 3019, 2950, 2931, 2863, 1755, 1370, 1219, 1094
; HRMS calcd for C13H16O2: m/e 204.11503, found 204.1147
; LRMS [EI] (rel %): 204.1 (2), 144.1 (20), 129.1 (7), 112.0 (6), 92.0 (100)
.
21.
The yield reported is that of the submitters and is based on the use of the pure
(E)-1-acetoxy-1,3-butadiene. It was found by the checkers that use of a mixture of the E, Z-isomers (as purchased from Aldrich Chemical Company, Inc.) led to an average yield of
73%.
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
7α-Acetoxy-(1Hβ, 6Hβ)-bicyclo[4.4.1]undeca-2,4,8-triene:
Bicyclo[4.4.1]undeca-3,7,9-triene-2-ol, acetate, endo- (±)- (12); (129000-83-5)
Tricarbonyl(η6-cycloheptatriene)chromium(0):
Chromium, tricarbonyl (1,3,5-cycloheptatriene)- (8);
Chromium, tricarbonyl[(1,2,3,4,5,6-η)-1,3,5-cycloheptatriene]- (9); (12125-72-3)
Acetonitrile (8,9), (75-05-8)
Chromium hexacarbonyl: HIGHLY TOXIC:
Chromium carbonyl (8);
Chromium carbonyl (OC-6-11)- (9); (13007-92-6)
Cycloheptatriene:
1,3,5-Cycloheptatriene (8,9); (544-25-2)
Tris(acetonitrile)chromium tricarbonyl:
Chromium, tris(acetonitrile)tricarbonyl- (8,9); (16800-46-7)
(E)-1-Acetoxy-1,3-butadiene:
1,3-Butadiene-1-ol acetate, (E)- (9); (35694-20-3)
Crotonaldehyde:
Crotonaldehyde, (E)- (8);
2-Butenal, (E)- (9); (123-73-9)
Isopropenyl acetate:
1-Propen-2-ol, acetate (8,9); (108-22-5)
p-Toluenesulfonic acid (8);
Benzenesulfonic acid, 4-methyl- (9); (104-15-4)
Cupric acetate monohydrate:
Acetic acid, copper(2+) salt, monohydrate (8,9); (6046-93-1)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved