Org. Synth. 2000, 77, 176
DOI: 10.15227/orgsyn.077.0176
BIS(PINACOLATO)DIBORON
[
2,2'-Bi-1,3,2-dioxaborolane, 4,4,4',4',5,5,5',5'-octamethyl-
]
Submitted by Tatsuo Ishiyama, Miki Murata, Taka-aki Ahiko, and Norio Miyaura
1
.
Checked by Glenn C. Micalizio and William R. Roush.
1. Procedure
Caution! All the operations should be carried out in a well-ventilated hood, since bromoborane derivatives fume in air and are rapidly hydrolyzed with the evolution of considerable heat.
A.
Tris(dimethylamino)borane
(Note 1). A
2-L, three-necked flask equipped with a
mechanical stirrer,
dropping funnel, and a
dry ice-cooled reflux condenser connected to a
nitrogen source and a bubbler is flushed with
nitrogen
(Note 2). The flask is charged with
800 mL of pentane
(Note 3) and
218 g (4.84 mol) of dimethylamine
(Note 4), and cooled to ca. −30°C with a dry
ice-methanol bath. A solution of
201 g (0.801 mol) of boron tribromide
(Note 5) in
400 mL of pentane
is added dropwise through the
addition funnel over 3 hr to the vigorously stirred solution while maintaining the bath-temperature at −20°C to −10°C
(Note 6). As soon as the addition is begun, a white precipitate of
dimethylamine hydrobromide
appears. The temperature is allowed to rise to ambient temperature without removing the cooling bath, and the slurry is stirred for 16 hr at room temperature. The precipitate is removed by filtration through a
Celite pad on a glass-fritted filter funnel
(Note 7). The flask and filter cake are rinsed three times with
60 mL of pentane
. The
pentane solution is distilled to give
92.7 g (
81%) of
tris(dimethylamino)borane
as a colorless liquid, bp
44-45°C (12 mm), lit.
2 bp
39°C (10 mm)
(Note 8),
(Note 9).
B.
Bromobis(dimethylamino)borane
. A
500-mL, two-necked flask equipped with a
magnetic stirring bar, dropping funnel, and a distillation apparatus connected to a
nitrogen source and a bubbler is flushed with
nitrogen
(Note 2). The flask is charged with
100 mL of pentane
(Note 3) and
92.7 g (0.648 mol) of tris(dimethylamino)borane
, and cooled to −40°C (external bath temperature) with a dry ice-methanol bath. A solution of
81.3 g (0.324 mol) of boron tribromide
(Note 5) in
80 mL of pentane
is added dropwise to the stirred solution over a period of 1.5 hr maintaining the external bath temperature at −40°C. The cooling bath is removed and the solution is stirred at room temperature for 30 min. Distillation affords
172.8 g (
99%) of
bromobis(dimethylamino)borane
as a colorless liquid, bp
56-58°C (12 mm), lit.
3 bp
20-28°C (0.5 mm)
(Note 10).
C.
Tetrakis(dimethylamino)diboron.
A
500-mL, three-necked flask equipped with an
airtight mechanical stirrer, a dropping funnel, and a reflux condenser connected to a
nitrogen source and a bubbler is flushed with
nitrogen
(Note 2). The flask is charged with
78 mL of toluene
(Note 11) and
22.3 g (0.97 g-atom) of sodium
. The mixture is brought to reflux with an
oil bath and the
sodium is finely pulverized by vigorous stirring. A solution of
135.6 g (0.758 mol) of bromobis(dimethylamino)borane
in
55 mL of toluene
is added dropwise at a rate sufficient to maintain a gentle reflux over 45 min. Shortly after the addition is begun, a deep-blue precipitate appears
(Note 12). The suspension is heated at reflux for an additional 2.5 hr. The slurry is cooled to room temperature, and filtered through a Celite pad on a sintered-glass funnel
(Note 7). The flask and filter cake are rinsed three times with
50 mL of toluene
(Note 13). The yellow filtrate is concentrated under reduced pressure, and the residual oil is distilled under reduced pressure to give
54 g (
72%) of
tetrakis(dimethylamino)diboron
as a colorless liquid, bp
92°C (12 mm), lit.
3 bp
55-57°C (2.5 mm)
(Note 14).
D.
Bis(pinacolato)diboron
(1). A 2-L, three-necked flask fitted with a mechanical stirrer, dropping funnel, and a reflux condenser connected to a
nitrogen source and a bubbler is flushed with
nitrogen
(Note 2). To the flask are added
53.7 g (0.271 mol) of tetrakis(dimethylamino)diboron
and
510 mL of toluene
(Note 11), and then a solution of
64.4 g (0.545 mol) of pinacol
(Note 15) in
340 mL of toluene
. The flask is immersed in an
ice-water bath and a 5.4 M ethereal solution of
hydrogen chloride
(Note 16) (203 mL, 1.10 mol) is added dropwise during 2 hr. As soon as the addition is started, a white precipitate of
dimethylamine hydrochloride
appears. The slurry is stirred at room temperature for an additional 4 hr. The precipitate is removed by filtration in a
Büchner funnel with suction, and the filtrate is concentrated on a
rotary evaporator to give a white solid. The solid is dissolved in ca.
700 mL of pentane
and the remaining solid is again removed by filtration. The filtrate is washed three times with 500 mL of water and dried over anhydrous
magnesium sulfate
. The drying agent is removed by filtration and the filtrate is concentrated to ca. 150 mL. The flask is heated to dissolve the resulting precipitate, allowed to cool to room temperature, and then thoroughly chilled in a freezer (−30°C). The first crop is collected by filtration and washed twice with
30 mL of cold pentane
. The mother liquor is again concentrated to give another crop of crystals. The procedure is repeated two additional times. The combined crystals are dried under reduced pressure (0.1 mm) for 16 hr at room temperature to give
54.3 g (
79%) of
1 as colorless plates, mp
138°C, lit.
4 mp
138°C
(Note 17),
(Note 18).
2. Notes
1.
Tris(dimethylamino)borane is available from Aldrich Chemical Company, Inc.
It may also be synthesized from
boron trichloride.
2
2.
All glassware is predried in an
oven at 120°C for 1 hr, assembled while hot, and allowed to cool under a stream of
nitrogen.
3.
Pentane is distilled from
lithium aluminum hydride before use.
4.
Dimethylamine (bp
6°C) is condensed at −78°C into a 500-mL flask fitted with an inlet tube and a
nitrogen bubbler. The quantity of
dimethylamine in the flask is determined by periodic weighing.
Dimethylamine is either taken from a cylinder (Aldrich Chemical Company, Inc.) or from a mixture of an aqueous
50% solution of dimethylamine
and
potassium hydroxide.
5 Checkers condensed
dimethylamine directly into the
2-L reaction vessel with periodic weighing.
5.
Boron tribromide was purchased from Wako Pure Chemical Industries, Ltd. or Aldrich Chemical Company, Inc.
, and used without further purification.
6.
Caution! Addition at lower than −20°C leads to a violent reaction during warming up to −10-0°C.
7.
A large filter area is recommended. A 1-cm layer of Celite is pressed on a sintered-glass funnel (6 cm × 17 cm). The Celite is dried in an oven at 120°C for 12 hr. A 6-mm Teflon tube is used to connect the flask and the filter funnel through the
septa, and the stirred slurry is then transferred to the funnel with the aid of
nitrogen pressure. Inner pressure of the receiver flask and the funnel is leaked through oil bubblers. The checkers had difficulty with the filter clogging, so they used a
Schlenk filter apparatus instead.
8.
Tris(dimethylamino)borane is moisture sensitive.
1H NMR (CDCl
3) δ: 2.52 (s, 18 H)
.
9.
The checkers obtained a
63% yield of
tris(dimethylamino)borane. The checkers obtained bp
57°C (11 mm).
10.
Bromobis(dimethylamino)borane is moisture sensitive and fumes in air:
1H NMR (CDCl
3) δ: 2.75 (s, 12 H)
. The checkers obtained a
96% yield of material with bp
71°C (13 mm).
11.
Toluene is distilled from molten
sodium before use.
12.
The checkers observed an induction period of approximately 20 min followed by a vigorous exotherm. It was only after this exotherm occurred that any blue precipitate was observed in the reaction vessel.
13.
The residual solid containing unreacted
sodium is carefully treated with
ethanol.
14.
Tetrakis(dimethylamino)diboron is moisture sensitive.
1H NMR (CDCl
3) δ: 2.67 (s, 24 H)
. The checkers observed an unidentified impurity in the
1H NMR spectrum (δ 2.51, s). The checkers also obtained a slightly better yield (
72%) than reported by the submitters (
67%) when a freshly opened bottle of
sodium was used in this experiment.
15.
Pinacol was purchased from Tokyo Kasei Kogyo Co., Ltd. or Aldrich Chemical Company, Inc.
, and used without further purification.
16.
An ethereal solution of
hydrogen chloride is titrated with 0.1 M
sodium hydroxide before use.
17.
The submitters obtained a
91% yield of
bis(pinacolato)diboron.
18.
Crystalline
bis(pinacolato)diboron can be handled in air and stored in a capped bottle. The physical properties are as follows:
1H NMR (300 MHz, CDCl
3) δ: 1.25 (s, 24 H)
;
11B NMR (128.3 MHz, toluene) δ 30.61 (BF
3·Et
2O as external reference, δ 0.00)
;
13C NMR (100 MHz, CDCl
3) δ: 83.4, 24.9
; IR (KBr) cm
−1: 2978, 2930, 1372, 1289, 1189, 1177, 1127, 960, 850, 744, 660, 547
; high resolution mass spectrum, calcd for C
12H
24B
2O
4 [M
+], 254.1861, found 254.1867
. Anal. Calcd. for C
12H
24B
2O
4; C, 56.76; H, 9.53. Found: C, 56.66; H, 9.50.
Bis(pinacolato)diboron is now commercially available from Lancaster Synthesis Ltd., Callery Chemical Co., Aldrich Chemical Company, Inc., and Frontier Scientific Inc.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
This method is an adaption of the U. S. Borax Research group's procedure
3 that illustrates a practical and efficient method for the synthesis of tetra(alkoxo)diborons. Several (alkoxo)diborons, such as tetra(methoxo)-,
3 bis(catecholato)-,
6 and bis(pinacolato)diboron
4
(1), are synthesized from
tetrakis(dimethylamino)diboron. The diborons are excellent reagents for the synthesis of various organoboronic esters via the transition metal-catalyzed addition and cross-coupling reactions.
7,8,9,10,11,12,13,14,15
The
platinum(0) complexes catalyze the addition of
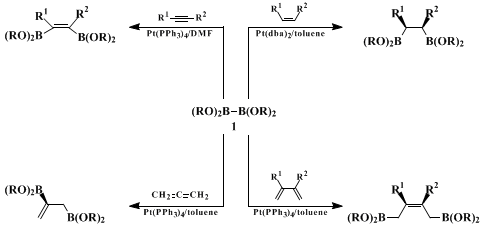
1 to unsaturated hydrocarbons (Scheme 1). The addition to alkynes,
7 alkenes,
8 1,3-dienes,
9 or allenes
10 stereoselectively provides cis-addition products.
The cross-coupling reaction of
1 with
palladium catalyst provides a convenient method for the synthesis of organoboronic esters from organic electrophiles (Scheme 2). Aromatic halides
11 and triflates
12 couple with
1 in the presence of PdCl
2(dppf) and
potassium acetate (KOAc) to give arylboronates in high yields. The procedure has a wider application over the conventional synthesis based on the addition of aryllithium or Grignard reagents to trialkyl borates, because the reaction tolerates various functional groups, e.g., -CO
2Me, -COMe, -CN, and -NO
2. Arylboronic acids and esters have been used for the synthesis of biaryls via the palladium-catalyzed cross-coupling reaction with aryl electrophiles. The use of diboron allows sequential, double cross-couplings in the same flask to provide biaryls (eq. 1).
12,13
Coupling with allyl acetates gives allyl boronates,
14 which exhibit a high diastereoselectivity in the intramolecular allyl boration of carbonyl compounds
15 (Scheme 2).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Bis(pinacolato)diboron:
2,2'-Bi-1,3,2-dioxaborolane, 4,4,4',4',5,5,5',5'-octamethyl- (10); (73183-34-3)
Dimethylamine (8);
Methanamine, N-methyl- (9); (124-40-3)
Tris(dimethylamino)borane:
Borane, tris(dimethylamino)- (8);
Boranetriamine, hexamethyl- (9); (4375-83-1)
Boron tribromide:
Boron bromide (8);
Borane, tribromo- (9); (10294-33-4)
Bromobis(dimethylamino)borane:
Borane, bromobis(dimethylamino)- (8);
Boranediamine, 1-bromo-N,N,N',N'-tetramethyl- (9); (6990-27-8)
Tetrakis(dimethylamino)diboron:
Diborane(4), tetrakis(dimethylamino)- (8);
Diborane(4)tetramine, octamethyl- (9); (1630-79-1)
Sodium (8,9); (7440-23-5)
Pinacol:
2,3-Butanediol, 2,3-dimethyl- (8,9); (76-09-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved