Org. Synth. 2002, 78, 91
DOI: 10.15227/orgsyn.078.0091
PREPARATION AND USE OF N,N'-DI-BOC-N"-TRIFLYLGUANIDINE
[
Carbamic acid, [[(trifluoromethyl)sulfonyl]carbonimidoyl]bis-,
bis(1,1-dimethylethyl) ester
]
Submitted by Tracy J. Baker, Mika Tomioka, and Murray Goodman
1
.
Checked by Dustin J. Mergott and William R. Roush.
1. Procedure
A.
N,N'-di-Boc-N"-triflylguanidine.
A 250-mL,
two-necked, round-bottomed flask equipped with a 10-mL pressure-equalizing
dropping funnel sealed with a rubber septum, gas
inlet, and a large football-shaped Teflon-coated magnetic stirring
bar is purged with nitrogen
(Note 1).
The flask is charged with
N,N'-di-Boc-guanidine
(7.5 g, 29 mmol, Note 2),
dichloromethane(100 mL,
Note 3), and
triethylamine
(5.0 mL, 36 mmol, Note 4). The temperature of the mixture is equilibrated to −78°C
using a dry ice/isopropyl alcohol bath.
Triflic
anhydride (5.9 mL, 35 mmol, Note 5) is added dropwise through the dropping
funnel over a period of 20 min, and the resulting mixture is allowed to
warm to −20°C over 4 hr (Notes 6, 7). A 2 M aqueous sodium
bisulfate
solution is added to the mixture at −20°C, such
that the reaction temperature does not rise above −10°C, and the resulting layers
are stirred vigorously for 5 min (longer stir times lead to decreased yields). The
layers are immediately separated, and the aqueous phase is extracted with
dichloromethane (3 × 50 mL).
The combined organic layers are washed with 2 M aqueous sodium
bisulfate (80 mL), brine
(50 mL), dried (MgSO4), filtered and concentrated
under reduced pressure. The crude material is purified by flash column chromatography
(Note 8) and dried under reduced pressure to afford
N,N'-di-Boc-N"-triflylguanidine
(10 g, 90%, mp 124°C,
Notes 9, 10).
B.
N,N'-Bis(tert-butoxycarbonyl)-N"-benzylguanidine.
An oven-dried, 50-mL, round-bottomed flask equipped with a
magnetic stirring bar is charged with
N,N'-di-Boc-N"-triflylguanidine
(1.00 g, 2.55 mmol)
and
dichloromethane
(13 mL, Notes 11, 12).
Benzylamine
(0.31 mL, 2.8 mmol, Note 13) is added in one portion via syringe at room temperature.
After 30 min, the mixture is transferred to a 60-mL separatory funnel
and washed with 2 M aqueous sodium bisulfate
(10 mL) and saturated sodium
bicarbonate (10 mL). Each aqueous layer is
extracted with
dichloromethane (2
× 10 mL). The combined organic phases are washed with brine (10 mL), dried (MgSO4),
filtered and concentrated under reduced pressure to afford
N,N'-di-Boc-N"-benzylguanidine
in quantitative yield (0.89 g,
Notes 14, 15).
2. Notes
1.
All glassware was flame-dried and cooled in a desiccator charged
with anhydrous calcium sulfate. Once completely cooled, the glassware was quickly
assembled and purged with
nitrogen.
2.
N,N'-di-Boc-guanidine
[1,3-bis(tert-butoxycarbonyl)guanidine, 98%]
was purchased from Aldrich Chemical Company, Inc.
(catalog #: 49,687-1) and used as received. Alternatively,
N,N'-di-Boc-guanidine
can be easily synthesized from
guanidine hydrochloride and
di-tert-butyl
dicarbonate according to the literature procedure.
2
3.
Dichloromethane
(Fisher Scientific Company) was predried with
calcium
chloride and freshly distilled under
argon from
calcium
hydride prior to use.
4.
Triethylamine (Aldrich
Chemical Company, Inc.) was predried with
potassium
hydroxide and freshly distilled under
argon from
calcium
hydride prior to use.
5.
Triflic anhydride
(trifluoromethanesulfonic anhydride, ε 98%)
was purchased from Fluka Chemika
and used as received.
6.
The reaction mixture turned yellow-orange upon addition of
triflic
anhydride.
7.
The submitters allowed the reaction to warm to −5°C prior
to quenching with with bisulfate solution, and obtained the product in 90% yield.
They noted that if the reaction mixture was allowed to warm above −5°C or stir
longer than 4 hr,
N,N'-di-Boc-N"-triflylguanidine
degrades to
N-mono-Boc-N"-triflylguanidine. The checkers
obtained yields of 71-85% when this procedure was followed exactly. However, if the
reaction was quenched at −20°C, with care not to allow the internal temperature
to rise above −10°C during the quench or to stir longer than 5 min following
the quench, the checkers obtained yields of 93-96%.
8.
A column (5 cm in diameter) of
silica
gel (J. T. Baker, 233-400 mesh, 250 g, dry-packed)
was equilibrated with 20% hexanes in
dichloromethane. The crude
material dissolved in a minimal amount of
chloroform
was loaded on the column and eluted with 20% hexanes in
dichloromethane.
Fractions were collected in 15 × 160 mm test tubes.
9.
The compound has the following characteristics:
1H NMR (0.6 M, DMSO-d
6; 500 MHz)
δ: 1.46 (s, 18 H, CH
3), 11.06 (br s, 2 H, NH)
;
13C NMR (0.6
M, DMSO-d
6; 125 MHz) δ: 27.5 (6C), 83.4 (2C),
119.1 (q, J
CF = 320 Hz), 150.1 (2C), 152.3
; IR (neat film/NaCl plate) cm
−1: 1202,
1343, 1557, 1622, 1739, 1788,
3300
; FAB-MS m/z (relative
intensity) 414 (M+Na)+, 392 (M+H)+, 336, 280,
236
. Anal. Calcd for C
12H
20F
3N
3O
6S:
C, 36.83; H, 5.15; N, 10.74; F, 14.56; S, 8.19. Found: C, 36.93; H, 5.21; N, 10.66;
F, 14.80; S, 8.33.
10.
The submitters report that the production scale of
N,N'-di-Boc-N"-triflylguanidine
can be increased ten-fold as long as Note 7 is followed.
11.
Reagent grade
dichloromethane may be used
without further purification.
12.
Guanidinylations of less reactive amines may require
triethylamine
(1.1 eq.) freshly distilled from
calcium hydride.
13.
Benzylamine (Aldrich Chemical
Company, Inc., 99%) is stored over
potassium
hydroxide and used without further purification.
14.
The spectral data of
N,N'-di-Boc-N"-benzylguanidine
matched that reported in the literature.
3
The sample has the following characteristics:
1H NMR (CDCl
3, 500 MHz) δ: 1.42
(s, 9 H, CH
3), 1.45 (s, 9 H, CH
3), 4.57
(d, 2 H, J = 5.2, CH
2
Ph), 7.21-7.20 (m, 5 H,
arom.), 8.55 (br s, 1 H, NH), 11.50 (br s, 1 H, NH)
;
13C NMR (CDCl
3,
125 MHz) δ: 28.0 (3C), 28.2 (3C), 45.0,
79.3, 83.1, 127.5, 127.7 (2C),
128.7 (2C), 137.1, 153.1, 156.0,
163.5
; IR (KBr) cm
−1:
1560, 1626, 1654, 1741
;
FAB-MS m/z (relative intensity) 350
(M+H)+, 238, 194, 91
; high
resolution mass spectrum, calcd for C
18H
27N
3O
4:
m/z 349.2002, found 349.1997.
The product was greater than 95% pure as determined by
1H NMR spectral
analysis.
15.
The production of
N,N'-di-Boc-N"-benzylguanidine
can be increased ten-fold or more.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Both natural and nonnatural guanidine-containing molecules have important biological
activity ranging from antimicrobial, antiviral, and antihypertensive to neurotoxic.
4 Hence, the conversion
of amines to guanidines has been a significant synthetic endeavor for many years.
The present method, using
N,N'-di-Boc-N"-triflylguanidine
for the guanidinylation of amines is the most efficient and general approach for most
applications in solution and on solid phase.
2,
5
To date, the most commonly used reagents for the guanidinylation of amines are
derivatives of protected thioureas in the presence of the Mukaiyama reagent,
3 pyrazole-1-carboxamidines,
6
and S-alkylisothioureas.
7 Protected
thioureas in conjunction with the Mukaiyama reagent have displayed the most versatile
usage. This combination has been successful in the conversion of sterically demanding
and resin-bound amines to protected guanidines.
3 However,
this method is limited to the use of highly polar aprotic solvents such as dimethylformamide
because of the solubility properties of the Mukaiyama reagent. Guanidinylations with
pyrazole-1-carboxamidines,
6 and S-alkylisothioureas
7 are sluggish in comparison to
N,N'-di-Boc-N"-triflylguanidine
and are incompatible with solid phase application.
The simple preparation of
N,N'-di-Boc-N"-triflylguanidine
from a commercially available source and its straightforward isolation make this reagent
extremely attractive. Guanidinylation using
N,N'-di-Boc-N"-triflylguanidine
is effective for amines both in solution and on solid phase.
2,
5 These reactions may be carried out in a variety of
solvents with
dichloromethane
and
chloroform
being the
most common; however, reaction rates slow with increase in solvent polarity. The use
of protected thioureas with the Mukaiyama reagent seems to be superior for guanidinylations
of sterically hindered and less reactive amines, but simple product isolation and
experimental setup of the
N,N'-di-Boc-N"-triflylguanidine
method make this the reagent of choice for most applications. Some representative
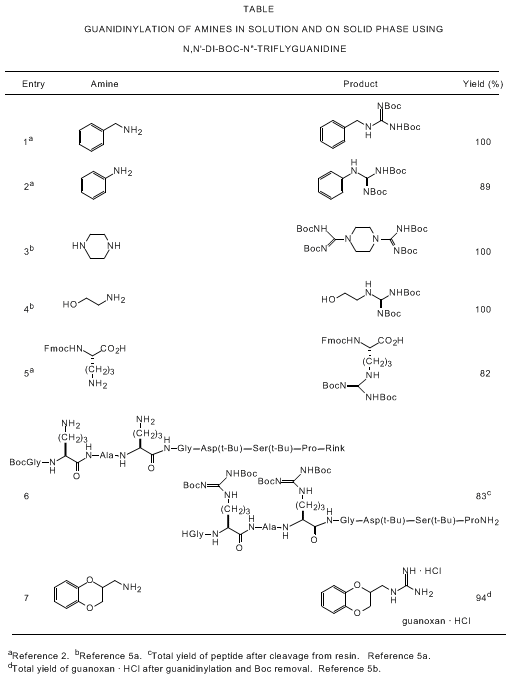
examples are compiled in the Table.
2,
5
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
N,N'-Di-Boc-N"-triflylguanidine:
N,N'-Bis(tert-butoxycarbonyl)-N"-trifluoromethanesulfonylguanidine:
Carbamic acid, [[(trifluoromethyl)sulfonyl]carbonimidoyl]bis-, bis(1,1-dimethylethyl)
ester (14); (207857-15-6)
N,N'-Di-Boc-guanidine:
1,3-Bis(tert-butoxycarbonyl)guanidine:
Carbamic acid, carbonimidolybis-, bis(1,1-dimethylethyl) ester
(13); (154476-57-0)
Triflic anhydride:
Methanesulfonic acid,
trifluoro-, anhydride (8,9); (358-23-6)
N,N'-Di-Boc-N"-benzylguanidine:
N,N'-Bis(tert-butoxycarbonyl-N"-benzylguanidine:
Carbamic acid, [[(phenylmethyl)imino]methylene]bis-, bis(1,1-dimethylethyl)
ester (13); (145013-06-5)
Benzylamine (8);
Benzenemethanamine
(9); (100-46-9)
Guanidine hydrochloride:
Guanidine, monohydrochloride
(9); (50-01-1)
Di-tert-butyl dicarbonate:
Formic acid, oxydi-,
di-tert-butyl ester (8);
Dicarbonic acid, bis(1,1-dimethylethyl)
ester (9); (24424-99-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved