Org. Synth. 2002, 79, 11
DOI: 10.15227/orgsyn.079.0011
1,2-METALLATE REARRANGEMENT: (Z)-4-(2-PROPENYL)-3-OCTEN-1-OL
[
3-Octen-1-ol, 4-(2-propenyl)-, (Z)-
]
Submitted by Krzysztof Jarowicki, Philip J. Kocienski, and Liu Qun
1
.
Checked by Christopher L. Franklin and Stephen F. Martin.
1. Procedure
Caution! tert-Butylithium is extremely pyrophoric and must not be allowed to come into contact with the atmosphere. This reagent should only be handled by individuals trained in its proper and safe use. It is recommended that transfers be carried out by using a 20-mL or smaller glass syringe filled to no more than 2/3 capacity, or by cannula. For a discussion of procedures for handling air-sensitive reagents, see Aldrich Technical Bulletin AL-134. [Note added August 2009]
A.
2,3-Dihydro-5-furyllithium
.
A
250-mL, three-necked, round-bottomed flask
(Note 1)
equipped with a
thermometer,
Teflon-coated magnetic
stirring bar
(Note 2) and a
nitrogen inlet
is charged with a solution of
2,3-dihydrofuran
(3.15 g, 3.4 mL, 45 mmol)
(Note 3) in
tetrahydrofuran
(THF, 6.13 g, 6.9 mL, 90 mmol)
(Note 4). The magnetically stirred solution is cooled in a
liquid
nitrogen-ethyl acetate cooling bath to an internal temperature of −85°C
whereupon
tert-butyllithium
(26.5 mL,
45 mmol, 1.7 M solution in pentane)
(Note 5) is added dropwise via a syringe during 10 min
(Note 6). After the addition the yellow semi-solid mixture is placed in
an
ice bath, stirred at 0-3°C for 30 min and diluted with
20 mL of diethyl ether
(Note 4) to give a thick yellow suspension of
2,3-dihydro-5-furyllithium
.
2
B.
Lithium dibutylcuprate
. A
250-mL, three-necked, round-bottomed flask, equipped with a
thermometer, Teflon-coated magnetic stirring bar
and a nitrogen inlet is charged with freshly recrystallized
copper bromide-dimethyl sulfide complex
(10.2 g, 49.5 mmol)
(Note 7)
and anhydrous diethyl ether (85
mL). To this suspension, cooled to −80°C in a liquid
nitrogen-ethyl acetate bath, is added dropwise via syringe during 20 min
a solution of
n-butyllithium (44.4
mL, 99 mmol, 2.23 M solution in hexanes) (Notes
5 and 8).
When the addition is complete, the reaction mixture is placed in an ice
bath and stirred at 0-3°C for 6 min to give a brown solution of
lithium dibutylcuprate
.
C.
(Z)-4-(2-Propenyl)-3-octen-1-ol
. The solution
of
lithium dibutylcuprate
is cooled to −80°C and transferred by cannula (Note 9)
under nitrogen pressure over 10 min to the stirred suspension
of
2,3-dihydro-5-furyllithium
cooled to −85°C (Note 10). After the transfer, the flask
containing the cuprate is washed with
5 mL
of diethyl ether
. The temperature of the light brown
suspension is allowed to rise slowly to 0°C over 3 hr (Note 11)
and stirred at 0-3°C for 30 min. The brown solution is cooled to −80°C and a
solution of
allyl bromide (17.9
g, 12.8 mL, 148 mmol)
(Note 3)
in
diethyl ether (20 mL)
is added dropwise during 10 min via syringe (Note 12). The black
mixture is left in the cooling bath to warm gradually to rt overnight (12 hr). The
resultant dark gray suspension is treated with an aqueous
saturated solution of ammonium chloride (50 mL)
and aqueous ammonia (20 mL,
30% in water). The organic layer is separated and the water layer extracted
with ether (2 × 75 mL). The combined
organic extracts are dried with
sodium sulfate
,
filtered, and concentrated under reduced pressure using a cold water bath
(10-20°C). The crude product (light brown oil) is purified by flash chromatography
on silica (150 g) (Note 13) eluting first with hexanes-diethyl
ether (5:1, 500 mL followed by 1:2, 700 mL).
The residue obtained from evaporation of the second fraction is distilled using a
Kugelrohr oven to give the product as a colorless oil (6.39 g, 84%),
bp 150°C (bath)/0.01 mm) (Notes 14, 15, 16).
2. Notes
1.
All glassware was dried for at least 8 hr at 80°C, assembled hot,
flame-dried, and allowed to cool under
nitrogen.
2.
Because of the semi-solid nature of the reaction mixture the use
of a
large football stirring bar (19 × 51 mm) or a
mechanical
stirrer is recommended.
3.
2,3-Dihydrofuran
was purchased from Aldrich Chemical Company, Inc.
,
and freshly distilled from
calcium hydride
under
nitrogen.
4.
Tetrahydrofuran
was distilled from
sodium/benzophenone
ketyl
or
potassium/benzophenone
under
nitrogen.
5.
tert-Butyllithium
was purchased from Aldrich Chemical Company, Inc.
,
and titrated using
1,3-diphenylacetone tosylhydrazone
.
3
6.
During the addition the temperature should not be allowed to rise
above −68°C.
7.
Commercial copper bromide
or its dimethyl sulfide complex contains impurities that are deleterious to the reaction.
Therefore, the
copper(I) bromide-dimethyl sulfide
complex
is prepared according to the method of House
4 from
copper(I) bromide
generated by reduction
of
copper(II) bromide (Aldrich
Chemical Company, Inc., 99%) with
sodium sulfite
.
5
Best results are obtained using
copper(I) bromide-dimethyl sulfide complex
freshly recrystallized according to the following procedure. A
100-mL conical
flask equipped with a
condenser and a
nitrogen
inlet is charged with
copper(I) bromide-dimethyl
sulfide complex (15 g).
Anhydrous
dimethyl sulfide
(50 mL) is added via syringe and the mixture heated gently
until all the solid dissolves. The heating bath is removed and
pentane
(25 mL) is added to the warm solution. The solution is
cooled in an
ice bath until crystallization is complete. The
product is collected by filtration under
nitrogen using a
sintered
glass funnel. The complex is washed with
7.5
mL of dimethyl sulfide-pentane
solution (2:1, v/v) and dried at rt under a stream of
nitrogen
for 1 hr to give
11.35 g of pure
copper(I) bromide-dimethyl sulfide complex
as a white solid.
8.
During the addition, the temperature should not be allowed to
rise above −58°C.
9.
Because of the viscosity of the solution the size of the cannula
is important. A
cannula with an internal diameter of 2 mm was
made from stainless steel HPLC tubing.
10.
During the addition the temperature should not be allowed to
rise above −76°C.
11.
It is important to increase the temperature slowly to minimize
the formation of by-products. The checkers found that this was most easily performed
using a
dry ice-acetone bath.
12.
During the addition the temperature should not be allowed to
rise above −30°C, and the mixture turns black.
13.
MN Kieselgel 60, 230-400 mesh, purchased from Machery-Nagel GmbH
& Co. was used. The checkers used ICN 32-63 D 60 Å, purchased from ICN Pharmaceuticals,
Inc.
14.
The spectral properties are as follows:
1H NMR (400 MHz, CDCl
3) δ:
0.90 (t, 3 H, J = 7.2), 1.24-1.44 (comp m, 4 H), 1.64
(br s, 1 H), 2.02 (t, 2 H, J = 7.2), 2.31 (dt, 2 H, J =
6.6, 7.1), 2.81 (d, 2 H, J = 6.5), 3.64 (t, 2 H, J = 6.5),
5.00 (ddt, 1 H, J = 1.5, 1.7, 10.0), 5.04 (dq, 1 H, J = 1.5, 17.1),
5.22 (t, 1 H, J =7.3), 5.76 (ddt, 1 H, J = 6.5, 10.2, 17.0)
;
13C NMR (100
MHz, CDCl
3) δ: 14.1, 22.6, 30.4,
31.5, 35.0, 37.0, 62.7, 115.3,
121.1, 136.4, 140.8
; IR (neat) cm
−1: 3340, 3072,
2928, 2866, 1631, 1456, 1052,
913
; MS (CI+) m/z:
169.1586 (C
11H
20O + H requires 169.1592),
169, 151, 127 (base), 109
;
Anal. Calcd. for C
11H
20O: C, 78.51; H, 11.98%. Found C, 78.43;
H, 11.83%.
15.
Capillary gas chromatography indicated a purity of 94%: DB 225
(30 m × 0.32 mm × 0.25 μm); 100°C (2 min) temperature programmed at 8°C/min to
160°C; He carrier 2.3 mL/min.
16.
The submitters have found recently that the same result can be obtained using
1 equiv of dihydrofuran, 1.1 equiv of butyllithium, and 1.1 equiv of commercial copper(I) cyanide.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
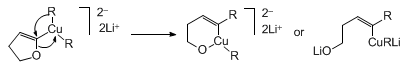
3. Discussion
The preparation of
(Z)-4-(2-propenyl)-3-octen-1-ol
described here illustrates the use of 1,2-metallate rearrangements of metal carbenoids
for the stereoselective synthesis of trisubstituted alkenes.
6
1,2-Metallate rearrangements of boronate carbenoids are well known to proceed with
inversion of stereochemistry at sp
3 hybridized carbon.
7 Examples
involving cuprates,
8
manganates
9
and zincates
10
are also known. The present reaction, a rare example of a 1,2-metallate rearrangement
involving inversion of stereochemistry at sp
2 hybridized carbon, was discovered
during an investigation of the Cu(I)-catalyzed ring scission of
2,3-dihydro-5-furyllithium
by
butyllithium
first described by Fujisawa and co-workers.
11
A mechanism involving the 1,2-metallate rearrangement of a higher order organocuprate
intermediate has been postulated.
12
The reaction has broad scope: 5-, 6-, and 7-membered ring metallated enol ethers participate
equally well as do organocuprates derived from MeLi, PhLi, sec-BuLi, tert-BuLi, Me
3SnLi,
and PhMe
2SiLi among others. The reaction also works with Grignard reagents.
13 Some examples are
given in the Table.
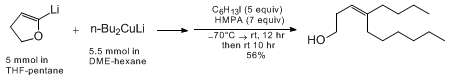
The final step in the sequence can be accomplished with less reactive halides such
as
hexyl iodide
with two
modifications to the reaction conditions: the lithium dialkylcuprate must be generated
in
1,2-dimethoxyethane
instead
of
diethyl ether
and HMPA
must be added along with the alkylating agent as illustrated in the following example:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(Z)-4-(2-Propenyl)-3-octen-1-ol:
3-Octen-1-ol,
4-(2-propenyl)-, (Z)- (12); (119528-99-3)
2,3-Dihydro-5-furyllithium:
Lithium, (4,5-dihydro-2-furanyl)-
(9); (75213-94-4)
2,3-Dihydrofuran:
Furan, 2,3-dihydro-
(8,9); (1191-99-7)
tert-Butyllithium:
Lithium, tert-butyl-
(8);
Lithium, (1,1-dimethylethyl)- (9); (594-19-4)
Copper (I) bromide-dimethyl sulfide complex:
Copper,
bromo[thiobis[methane]]- (9); (54678-23-8)
Allyl bromide:
1-Propene, 3-bromo-
(8,9); (106-95-6)
Dimethyl sulfide:
Methyl sulfide
(8);
Methane, thiobis- (9); (75-18-3)
Butyllithium:
Lithium, butyl-
(8,9); (109-72-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved