Org. Synth. 1927, 7, 50
DOI: 10.15227/orgsyn.007.0050
HYDROGEN CYANIDE (ANHYDROUS)
[Hydrocyanic acid]
Submitted by K. Ziegler
Checked by Henry Gilman and L. C. Heckert.
1. Procedure
A
5-l. round-bottomed flask, set up in a good
hood (Note 1), is fitted with a
three-holed rubber stopper that holds
two 250-cc. separatory funnels, A and B (Fig. 18). A small
funnel F (about 3–4 cm. in diameter) is suspended directly under the outlets of the two separatory funnels and is attached to the rubber stopper by a loop of stiff copper wire. The discharge tube of the funnel is bent in the shape of a U so that its end is about 1 cm. below the top of the funnel. In the third hole of the rubber stopper is inserted an
inclined glass tube, C, of about 10-mm. internal bore and approximately 50 cm. long. A
mercury safety vent, D, is sealed in the side of this tube. C acts as an
air condenser and leads to an
empty gas bottle, E, of about 250-cc. capacity and then to
three large U-tubes, G, H, and I, filled with anhydrous calcium chloride. The gas bottle and the U-tubes are contained in a
water bath, W, warmed to 30–40°. The last
calcium chloride tube is fitted with a
three-way glass stopcock so that the gaseous
anhydrous hydrogen cyanide may be used directly or diverted to an efficient condenser for liquefaction. The
condenser is a glass coil of 4–5 mm. bore and about 50 cm. long that is surrounded by ice
(Note 2) contained in a percolator arrangement like that described on
p. 117.
Fig. 18.
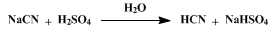
One of the separatory funnels is filled with dilute sulfuric acid prepared by the careful addition of 392 g. (213 cc., 4 moles) of concentrated sulfuric acid to 830 cc. of water. The other separatory funnel is filled with a solution of 203 g. (4 moles) of commercial sodium cyanide (about 96 per cent) dissolved in sufficient water to make 500 cc. of solution. Evolution of hydrogen cyanide takes place on the simultaneous addition of the two solutions. Practically all the reaction occurs in the funnel, F, and the sodium bisulfate solution continuously drains into the flask so that fresh solutions are always present. The solution in the funnel remains clear as long as sufficient sulfuric acid is present. An excess of sodium cyanide colors the solution yellow and leads to the formation of a muddy brown precipitate (Note 3). By adjusting the flow of solutions the rate of evolution is easily controlled, and the preparation requires no attention beyond that involved in the occasional replenishment of the solutions in the separatory funnels. The last part of the hydrogen cyanide can be driven from the apparatus by boiling the bisulfate solution for a few minutes. The yield of acid melting at − 15° to − 14.5° is 100–105 g. (93–97 per cent of the theoretical amount) (Note 4) and (Note 5).
2. Notes
1.
Gattermann
1 recommends that the operator smoke during the preparation, for he found that a trace of
hydrogen cyanide is sufficient to give the tobacco smoke a highly characteristic flavor. This preliminary warning is useful in case of leaky apparatus or a faulty hood.
2.
It is essential that the coil be cooled with ice only. A freezing mixture causes solidification of the
hydrogen cyanide with consequent clogging of the apparatus.
3.
If the reaction is slowed up considerably or interrupted so that the solution becomes cool,
sodium bisulfate crystallizes both in the funnel and in the
generating flask.
4.
The
hydrogen cyanide is best kept over anhydrous
calcium chloride. In this way it remains clear and water-white for months; otherwise, it soon becomes yellow, owing to the formation of
azulmic acid. Concentrated
hydrochloric acid (two drops per 500 g. of hydrogen cyanide) has also been recommended as a stabilizer.
25.
If larger quantities of
hydrogen cyanide are desired, the apparatus may be modified as suggested by Lindemann
3 by using a
four-holed rubber stopper in the generating flask and fitting it with a
siphon tube by means of which the
sodium bisulfate solution can be removed from time to time. With this modification the generating flask need be of only 2- to 3-l. capacity.
Hydrogen cyanide may be prepared conveniently, probably in lower yields than in the procedure described, by adding the saturated
sodium cyanide solution one-half inch below the surface of
50 per cent sulfuric acid contained in a flask. The residual amount is expelled by warming the flask on a
steam bath. The apparatus is the same as that used for generating
hydrogen chloride (p. 293) by passing aqueous
hydrochloric acid into
sulfuric acid (W. W. Hartman, private communication).
3. Discussion
Hydrogen cyanide can be prepared by the action of
sulfuric acid on
potassium ferrocyanide,
1 potassium cyanide4 or
sodium cyanide.
5 The procedure described is based on the methods of Ziegler
5 and Lindemann.
5
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
azulmic acid
calcium chloride (10043-52-4)
sulfuric acid (7664-93-9)
hydrogen chloride,
hydrochloric acid (7647-01-0)
sodium cyanide (143-33-9)
hydrogen cyanide,
hydrocyanic acid (74-90-8)
potassium cyanide (151-50-8)
sodium bisulfate (7681-38-1)
potassium ferrocyanide
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved