Org. Synth. 1943, 23, 20
DOI: 10.15227/orgsyn.023.0020
CYCLOPROPYL CYANIDE
[Cyclopropane, cyano-]
Submitted by M. J. Schlatter
Checked by R. L. Shriner and Chris Best.
1. Procedure
The apparatus shown in
Fig. 10 is assembled in a good
hood.
One liter of liquid ammonia and
0.5 g. of hydrated ferric nitrate are placed in the
2-l. three-necked flask A, which is equipped with a
stirrer and a
special reflux condenser cooled with Dry Ice. This condenser is attached to a
soda-lime tower which is connected to a source of compressed air through the T-tube
C. Over a period of about 45 minutes,
92 g. (4 gram atoms) of clean sodium shavings is added to the liquid
ammonia, and the mixture is stirred until the blue color disappears (1–2 hours).
Fig. 10.
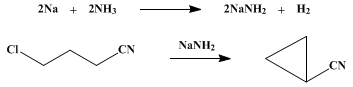
In the similarly equipped
5-l. three-necked flask B are placed
1.5 l. of liquid ammonia and
440 g. (4.25 moles) of γ-chlorobutyronitrile [Org. Syntheses Coll. Vol. 1, 156 (1941)]. The flasks are connected by means of the 12-mm. glass tube
D reaching to the bottom of the
sodamide flask A and extending 1 cm. through the
rubber stopper in one neck of flask
B. Vigorous stirring is maintained in both flasks while the
sodamide suspension is slowly forced over into the reaction flask in small portions
(Caution! (Note 1)) by means of air pressure applied through
C; the rate of addition is controlled by placing the finger over the by-pass in the T-tube. At first the reaction is violent, and only small amounts of the
sodamide solution should be added. The addition is continued at such a rate that the total time required for addition of all the
sodamide solution is 1–1.5 hours. The sodamide flask is rinsed with
300 ml. of liquid ammonia, and the washings are added to the reaction mixture, after which stirring is continued for 2 hours. During the second hour, addition of Dry Ice to the reflux condenser is discontinued, and the
ammonia is permitted to evaporate slowly. At the end of the 2-hour stirring period, the inlet tube is replaced by a
dropping funnel and
1 l. of dry ether is slowly added
(Note 2). The reaction mixture is quickly filtered through a
sintered-glass funnel, and the filter cake is washed with two
200-ml. portions of dry ether (Note 3). The
ammonia and
ether are removed by distillation on a
water bath through a
packed column (Note 4). The residue is then distilled through the column under the pressure of a
water pump (Note 5). The yield of
cyclopropyl cyanide boiling at
69–70°/80 mm. (
75–76°/95 mm.) is
149–152 g. (
52–53% based upon
γ-chlorobutyronitrile)
(Note 6) and
(Note 7). The pressure is then reduced, and the unchanged
γ-chlorobutyronitrile is collected at 93–96°/26 mm. It amounts to 52–62 g.
2. Notes
1.
The reaction is very vigorous, and addition of the
sodamide solutions in large portions must be avoided. The use of
stopcocks or pinch clamps to regulate the addition is not recommended.
2.
Very little
cyclopropyl cyanide is obtained unless the
ether is added
before complete evaporation of the
ammonia.
3.
The mixture may be permitted to stand at this point and the
ammonia allowed to evaporate spontaneously under a hood.
5.
It is desirable to interpose a
Dry Ice trap between the
receiver and the pump in order to prevent loss of the nitrile, and too low a pressure should not be used.
6.
The submitter reports that an equivalent number of moles of
γ-bromobutyronitrile or a mixture of
γ-chloro- and γ-bromobutyronitrile may be substituted for
γ-chlorobutyronitrile. If the bromo compound is used the reaction mixture should be refluxed 6 hours and it is necessary to filter off the
sodium bromide just before the final vacuum distillation.
7.
This yield is based upon the
γ-chlorobutyronitrile taken. When the recovered
γ-chlorobutyronitrile (52–62 g.) is taken into account, the yield of
cyclopropyl cyanide is about
60%.
3. Discussion
Cyclopropyl cyanide has been prepared by the repeated distillation of
γ-chlorobutyronitrile over powdered
potassium hydroxide,
1,2,3,4,5 or over a mixture of
sodium hydroxide and
alumina.
6 The preparation, on a small scale, of
cyclopropyl cyanide from
γ-chlorobutyronitrile by action of
sodium in liquid
ammonia, or of
sodium suspended in
ether, has been described.
7 The present directions are based upon those given by Schlatter.
8
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
alumina
γ-chloro- and γ-bromobutyronitrile
ammonia (7664-41-7)
ether (60-29-7)
sodium hydroxide (1310-73-2)
sodium bromide (7647-15-6)
potassium hydroxide (1310-58-3)
sodium (13966-32-0)
γ-Chlorobutyronitrile (628-20-6)
sodamide (7782-92-5)
ferric nitrate
Cyclopropyl cyanide,
Cyclopropane, cyano- (5500-21-0)
γ-bromobutyronitrile (5332-06-9)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved