Org. Synth. 1940, 20, 86
DOI: 10.15227/orgsyn.020.0086
SODIUM AMIDE
Submitted by F. W. Bergstrom
Checked by C. F. H. Allen and C. V. Wilson.
1. Procedure
The apparatus is assembled as shown in
Fig. 27.
Ammonia gas from a commercial cylinder
(Note 1) enters the system at
K.
R is a
mercury trap which would serve as a safety valve if the system should become blocked by solidification of the amide owing to an accidental drop in temperature.
J is a U-tube containing just enough
mercury to seal the bend, and it serves to estimate the rate of
ammonia flow.
I is a
Kjeldahl trap which prevents any
mercury from being thrown into the fusion pot
A, which
(Note 2) is conveniently supported on a tripod set on bricks to raise it to a convenient height above the burner
M. Through the cover of the fusion pot passes an outlet tube
B, a
thermometer well T, and the combined inlet tube
CDE. The thermometer well is welded shut at the bottom and projects about 6 mm. below the wider inlet tube, to which it is welded at the top. A gland or packed joint
O, through which the inlet passes, is packed with a few turns of asbestos cord, the upper hexagonal nut being turned down with a wrench so that
ammonia will not escape past the packing and so that there will be sufficient resistance to hold
CDE in any position to which it may be raised
(Note 3). The rubber tubes
H, H' should be of sufficient length (5–7 cm.) to be very flexible and facilitate manipulation of the hot cover. The outlet tube
B is at least 10 mm. in diameter.
Fig. 27.
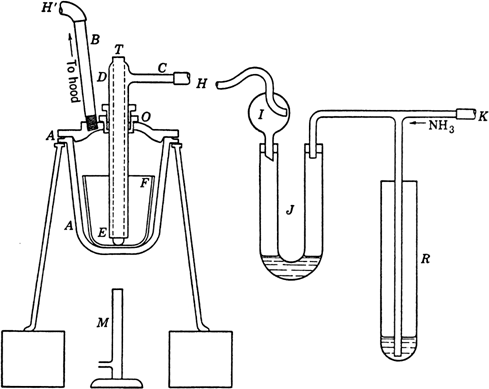
At the outset of the run the pot A, with the thermometer well in the position shown in the diagram, is heated to about 120° for 10 minutes in a slow stream of ammonia (Note 4). This serves to sweep the air and any traces of moisture from the system. The apparatus is then allowed to cool to 70–80°, the cover is removed, and a 250-ml. nickel crucible F is placed in the pot. The ammonia inlet CDE is raised to just above the top of the nickel crucible, in which is then placed approximately 175 g. (7.6 gram atoms) of clean sodium (Note 5). The pot is now heated with the full flame of the burner; the sodium melts in 5–10 minutes, whereupon the inlet tube CDE is pushed through the gland until it rests on the bottom of the crucible. When the temperature has reached 320°, the burner is turned down and adjusted to maintain the temperature at 350–360°. The ammonia flow is regulated so that the bubbles in J are just too rapid to count. After 3.5–4 hours (Note 6) and (Note 7), the temperature is lowered to about 320°, and the cover is lifted enough to see whether any unreacted metal remains; if none remains, the flame is removed and the crucible allowed to cool to 230–240°; at this temperature the burner is replaced and heating continued for 30 minutes to ensure removal of the bulk of the sodium hydride.
The burner is now extinguished, the ammonia shut off, and the pot cover removed by disconnecting at H, H'. The crucible is removed from the pot with tongs, and the molten amide is poured into a shallow iron tray, which has previously been heated to remove traces of moisture (Note 8). At this point it is essential to work rapidly to avoid solidification of the amide in the crucible (Note 9). As soon as the product has solidified sufficiently, the tray is transferred to a large desiccator to cool. When cold enough to handle, the tray is inverted on a clean heavy paper; the amide is removed by rapping the bottom of the pan and is at once transferred to convenient wide-mouthed bottles and covered with a petroleum fraction (Note 10) and (Note 11). The yields vary from 267 to 282 g. (90–95%) (Note 12), (Note 13), and (Note 14).
The sodium amide thus prepared is easily pulverized; it may be ground in a mortar under any hydrocarbon solvent. It is safer, though not necessary, where ether is to be used as a reaction medium, to grind the amide first under a hydrocarbon, the mixture being transferred to the reaction flask and then replaced by ether in the usual way (Note 15), (Note 16), and (Note 17).
2. Notes
1.
Ordinary commercial cylinders of ammonia are used; it is unnecessary to dry the gas.
2.
The fusion pot is obtainable on the market from the Denver Fire Clay Company (cast-iron crucible and cover, 0.25 gal., catalog No. 2136).
3.
The gland
O may be replaced by a sleeve or bushing and held in place by a set screw or by a clamp at any position desired.
4.
Considerable time is saved by using a
Meker or triple burner for raising the apparatus to reaction temperature, but an ordinary
Tirrill burner is sufficient for the reaction.
5.
The oxide coating or oil on commercial
sodium should be removed before using. It is more convenient to use approximately
175 g. of sodium than to cut this exact amount.
6.
The reaction time depends largely upon the rate at which the
ammonia is admitted. If the current is too rapid there will be considerable splashing, and much of the molten amide will collect in the
iron pot. Ordinarily the quantity of
sodium specified will react completely in the time indicated. The total time for a run is slightly under 6 hours, of which not more than 2–2.5 hours of actual attention are required.
7.
Any unreacted metal is easily visible as a globule, floating on the surface of the darker liquid. A flashlight aids in rapid inspection.
8.
A pan 2 cm. high and 13 cm. in diameter is suitable for a run of this size. Any oxide coat should be removed by heating to redness, cooling, and polishing with emery paper; otherwise the product is deeply colored where it comes into contact with the pan. The same pan may be used repeatedly without further treatment other than cleaning and drying.
9.
Some of the product invariably splashes out of the crucible onto the walls of the pot. If the quantity should be large (too rapid current of
ammonia) it can be poured out, but if small it is chipped out after cooling.
10.
Commercial "heptane" from petroleum, b.p.
90–100°, is preferable, but other fractions may be used. A 750-ml. bottle will hold the product from one run.
11.
Alternatively, the amide is allowed to cool completely in the nickel crucible in a slow current of
ammonia and removed when cold.
CDE is raised above the melt before cooling.
12.
The chief variation in the yield is due to loss by splashing; it is difficult to remove the amide that has soldified on the walls of the iron pot. Some loss is accounted for by the
sodium hydride carried away with the effluent gas.
13.
Runs of other sizes may be made in the same apparatus. With half the quantity of
sodium specified, temperature control demands much more attention. With larger quantities, the nickel crucible is dispensed with and the carefully cleaned pot is used. The checkers used
260–270 g. of sodium and averaged a yield of
94%; the reaction time was increased by only 30 minutes. By arranging two series of apparatus in parallel, but connected to the same cylinder of ammonia, one operator can prepare twice as much amide in almost the same time.
14.
The product is nearly white if the iron vessels are carefully cleaned but may be considerably on the gray side.
15.
Caution.
Sodium amide is a very reactive substance; it combines with
oxygen and reacts explosively with water. The submitters recommended keeping the amide in
sealed glass containers in an atmosphere of
ammonia. The checkers preferred the use of petroleum fractions for greater convenience in handling; they have kept specimens under this solvent for 3 years without appreciable loss in activity.
When exposed to the atmosphere,
sodium amide rapidly takes up moisture and
carbon dioxide. When exposed to only limited amounts, as in imperfectly sealed containers, products are formed which render the resulting mixture highly explosive.
1 The formation of oxidation products is accompanied by the development of a yellow or brownish color. If such a change is noticed, the substance should be destroyed at once. This is conveniently accomplished by covering with much
benzene,
toluene, or kerosene and slowly adding dilute
ethanol with stirring.
16.
After the preparation is completed, the cooled reactor should be dismantled and the parts immediately washed with
ethanol, care being taken that all traces of
sodium amide have been destroyed before water is brought into contact with any part of the equipment.
17.
The submitters have prepared
potassium amide in yields of
95% in the same manner, but maintaining the temperature at 350–360° for the entire run. The apparatus should be rinsed with an
ethanolbenzene mixture.
3. Discussion
Sodium amide has been prepared by the action of gaseous
2 or
liquid3 ammonia on
sodium, by the action of
ammonia on alloys of
sodium,
4 and by the electrolysis of a solution of
sodium cyanide5 in liquid
ammonia with a sodium amalgam electrode. A summary of the chemistry of alkali amides is given by Bergstrom and Fernelius.
1
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
ethanolbenzene
ethanol (64-17-5)
ammonia (7664-41-7)
Benzene (71-43-2)
ether (60-29-7)
sodium cyanide (143-33-9)
oxygen (7782-44-7)
mercury (7439-97-6)
carbon dioxide (124-38-9)
toluene (108-88-3)
sodium (13966-32-0)
sodium amide (7782-92-5)
heptane (142-82-5)
sodium hydride (7646-69-7)
potassium amide
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved