Org. Synth. 1965, 45, 69
DOI: 10.15227/orgsyn.045.0069
N-MONO- AND N,N-DISUBSTITUTED UREAS AND THIOUREAS
Submitted by Roy G. Neville
1 and John J. McGee
2.
Checked by William E. Parham and J. Kent Rinehart.
1. Procedure
DOI: 10.15227/orgsyn.045.0069
METHOD I
CYCLOHEXYLUREA
[Urea, cyclohexyl-]
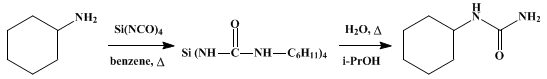
A solution of cyclohexylamine (39.7 g., 0.4 mole) (Note 1) in 100 ml. of anhydrous benzene (Note 2) is added slowly to a stirred solution (Note 3) of silicon tetraisocyanate (19.6 g., 0.1 mole) (Note 4) in 150 ml. of anhydrous benzene contained in a 1-l. round-bottomed flask. After the exothermic reaction has subsided, the mixture is heated at the reflux temperature for 30 minutes; the benzene is then removed using a rotary evaporator. Dilute isopropyl alcohol (200 ml.) (Note 5) is added to the residue, and the resulting mixture is heated at the reflux temperature for 30 minutes. The hot mixture is filtered through a 0.5-in. layer of Celite® contained in a coarse-grade sintered-glass funnel (Note 6). The gelationous silica is washed with two 75-ml. portions of acetone and is then pressed and drained. The combined filtrates are evaporated to dryness on a steam bath (Note 7). The crude cyclohexylurea (m.p. 185–191°, 55.0 g., 97% yield) is recrystallized from 220 ml. of isopropyl alcohol (Note 8) to give 37 g. (65%) of product, m.p. 192–193°. Concentration of the mother liquor affords about 9 g. (16%) of additional product which is less pure (m.p. 189–192°) (Note 9).
1. Procedure
DOI: 10.15227/orgsyn.045.0069
METHOD II
2,6-DIMETHYLPHENYLTHIOUREA
[Urea, 1-(2,6-dimethylphenyl)-2-thio-]
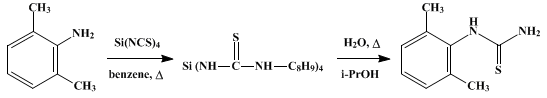
Silicon tetraisothiocyanate (26.0 g., 0.10 mole) (Note 10) is finely ground under 100 ml. of anhydrous benzene, and the mixture is quickly transferred to a 1-l. round-bottomed flask. The mortar and pestle are washed with two 25-ml. portions of anhydrous benzene, and the washings are added to the flask. A solution of 2,6-dimethylaniline (48.5 g., 0.4 mole) (Note 1) in 100 ml. of anhydrous benzene is added to the well-stirred mixture. The reaction is mildly exothermic. The mixture is heated at the reflux temperature for 30 minutes, and the benzene is then removed using a rotary evaporator. Dilute isopropyl alcohol (200 ml.) (Note 5) is added to the residue, and the resulting mixture is heated at the reflux temperature for 30 minutes. The mixture is then processed in exactly the same manner as described above for the preparation of cyclohexylurea. The crude 2,6-dimethylphenylthiourea (m.p. 193–197°, 71.3 g., 99% yield) is recrystallized from 280 ml. of isopropyl alcohol (Note 8) to give 50 g. (72%) of product, m.p. 201–202°. Concentration of the mother liquor affords 11 g.. (15%) of less pure product, m.p. 197–199° (Note 11).
2. Notes
1.
Cyclohexylamine and 2,6-dimethylaniline were obtained from Eastman Organic Chemicals and were redistilled prior to use.
2.
Benzene was dried over
sodium wire.
3.
The mixture becomes viscous; however, a
good magnetic stirrer is adequate. The checkers found it convenient to decrease the viscosity of the mixture by increasing the volume of
benzene from 100 ml. to 150–300 ml.4.
Silicon tetraisocyanate is prepared from
silicon tetrachloride and
silver cyanate or
lead cyanate.
3,45.
Dilute isopropyl alcohol is prepared by mixing the alcohol (180 ml.) with water (20 ml.). The use of more than about 10% water in the alcohol results in an intractable mass of gelatinous silica from which it is very difficult to separate a good yield of the urea.
6.
As gelatinous silica clogs the filter when too strong a suction is applied, it is best to carry out the filtration using very gentle suction. Only when almost all the liquid has passed through the filter is strong suction applied. The checkers used Hyflo Supercel
® as the filter aid, and a
600-ml. coarse-grade sintered-glass funnel.
7.
An
open dish or a rotary evaporator is satisfactory.
8.
Isopropyl alcohol is a good solvent to employ for recrystallizing most ureas; however, occasionally a mixture of alcohol and
benzene or pure
benzene is superior.
9.
This material is of sufficient purity for most purposes. If a purer product is required, the first crop of the
cyclohexylurea (
37 g.) is recrystallized from
135 ml. of isopropyl alcohol to yield
23 g. of product, m.p.
195.5–196.0°10.
Silicon tetraisothiocyanate is prepared from
silicon tetrachloride and
silver thiocyanate5,6 or, preferably,
ammonium thiocyanate.
6,7 The
silicon tetraisothiocyanate used by the checkers was slightly yellow; however, this did not affect the yield of product.
11.
This material is of sufficient purity for most purposes. If purer material is required, the first crop of
2,6-dimethylphenylthiourea (
50 g.) is recrystallized from
250 ml. of isopropyl alcohol to yield
41 g. of product, m.p.
203.5–204.0°.
3. Discussion
Cyclohexylurea has been prepared by the reaction of
cyclohexyl isocyanate with
gaseous ammonia8 or
ammonium hydroxide,
9 by thermal decomposition of
cyclohexyl allophanamide,
10 by treating
cyclohexylamine hydrochloride with an aqueous solution of
potassium cyanate,
11 by heating
nitrosomethylurea with
cyclohexylamine,
12 and by heating an ethanolic solution of
cyclohexylamine and
3,5-dimethyl-1-carbamylpyrazole.
13
2,6-Dimethylphenylthiourea has been synthesized by allowing
2,6-dimethylaniline hydrochloride to react with
ammonium thiocyanate.
14
4. Merits of the Preparation
These procedures are generally applicable to aliphatic, alicyclic, aralkyl, aromatic, and heterocyclic primary or secondary amines. The reactions fail or give poor yields with sterically hindered amines such as
2-trifluoromethylaniline,
2,6-dibromoaniline, and
diphenylamine. In general, however, excellent (95–100%) yields of N-mono- or N,N-disubstituted ureas or thioureas can be obtained by employing these versatile reactions which are, in most cases, superior to and supplement the methods conventionally employed for the synthesis of ureas and thioureas.
15 Because of the rapidity, ease, and excellent yields of these reactions,
silicon tetraisocyanate and tetraisothiocyanate (both of which are readily prepared
4,6) are likely to become standard reagents for the preparation of N-mono- and N,N-disubstituted ureas and thioureas. The submitters have employed these reagents to prepare large-scale (O.4
M) amounts of the following compounds (yields in parentheses):
benzylurea, m.p.
148° (
96%);
16 phenylurea, m.p.
147° (
95%);
17 t-butylurea, m.p.
182° (
95%);
18 N-(2-benzothiazolyl)urea, m.p.
>350° (
95%);
19,20 dibenzylthiourea, m.p.
140° (
95%);
21 t-butylthiourea, m.p.
168° (
98%).
22 In addition, the scope and limitation of the reactions of
silicon tetraisocyanate and tetraisothiocyanate have been investigated with more than fifty alkyl, aralkyl, aromatic, and heterocyclic primary and secondary amines.
23,24
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
N-MONO- AND N,N-DISUBSTITUTED UREAS AND THIOUREAS
silicon tetraisocyanate and tetraisothiocyanate
ammonia (7664-41-7)
Benzene (71-43-2)
ammonium thiocyanate (1762-95-4)
acetone (67-64-1)
sodium (13966-32-0)
isopropyl alcohol (67-63-0)
ammonium hydroxide (1336-21-6)
Phenylurea (64-10-8)
potassium cyanate (590-28-3)
diphenylamine (122-39-4)
Nitrosomethylurea
cyclohexylamine (108-91-8)
2,6-Dibromoaniline (608-30-0)
silver cyanate (3315-16-0)
Cyclohexylurea,
Urea, cyclohexyl- (698-90-8)
silicon tetraisocyanate (3410-77-3)
Urea, 1-(2,6-dimethylphenyl)-2-thio-,
2,6-Dimethylphenylthiourea (6396-76-5)
Silicon tetraisothiocyanate (6544-02-1)
2,6-dimethylaniline (87-62-7)
silicon tetrachloride (10026-04-7)
silver thiocyanate (1701-93-5)
cyclohexyl isocyanate (3173-53-3)
cyclohexyl allophanamide
cyclohexylamine hydrochloride (4998-76-9)
3,5-dimethyl-1-carbamylpyrazole (934-48-5)
2,6-dimethylaniline hydrochloride (21436-98-6)
2-trifluoromethylaniline (88-17-5)
benzylurea (538-32-9)
N-(2-benzothiazolyl)urea
dibenzylthiourea
t-butylurea (1118-12-3)
lead cyanate
t-butylthiourea (7204-48-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved