Org. Synth. 1976, 55, 32
DOI: 10.15227/orgsyn.055.0032
tert-BUTYLCYANOKETENE
[Butanenitrile, 2-carbonyl-3,3-dimethyl-]
Submitted by Walter Weyler, Jr., Warren G. Duncan, Margo Beth Liewen, and Harold W. Moore
1.
Checked by B. E. Smart and R. E. Benson.
1. Procedure
Caution! Benzene has been identified as a carcinogen; OSHA has issued emergency standards on its use. All procedures involving benzene should be carried out in a well-ventilated hood, and glove protection is required.
A. 2,5-Di-tert-butyl-5,6-dichloro-2-cyclohexene-1,4-dione. A 2-l. Erlenmeyer flask is charged with 110 g. (0.500 mole) of 2,5-di-tert-butyl-1,4-benzoquinone (Note 1) and 500 ml. of glacial acetic acid. The reaction flask is equipped with an efficient magnetic stirring bar, and chlorine gas is introduced through a safety trap into the well-stirred mixture (Note 2). During the course of the reaction, which should be completed in 35–40 minutes (Note 3), the mixture becomes homogeneous and warms to about 60°. The mixture is flushed with nitrogen to expel excess chlorine. During this process the product crystallizes from the solution; precipitation is completed by cooling the reaction mixture to 20°. The mixture is filtered, and the product is washed with 1 l. of water and air dried, yielding 112 g. (77%) of 2,5-di-tert-butyl-5,6-dichloro-2-cyclohexene-1,4-dione, m.p. 125–129° (Note 4).
B. 3-Chloro-2,5-di-tert-butyl-1,4-benzoquinone. A 2-l. Erlenmeyer flask is charged with a solution of 112 g. (0.385 mole) of 2,5-di-tert-butyl-5,6-dichloro-2-cyclohexene-1,4-dione in 800 ml. of diethyl ether. A solution of 28.4 g. (0.383 mole) of diethylamine in 50 ml. of ether is added in one portion to the vigorously swirled flask (Note 5). The reaction is instantaneous, resulting in a voluminous precipitate. The mixture is washed with two 1-l. portions of water, then with 500 ml. of aqueous saturated sodium chloride. The yellow ether solution is dried over anhydrous magnesium sulfate, filtered, and concentrated on a rotary evaporator, yielding 96–97 g. (98–99%) of 3-chloro-2,5-di-tert-butyl-1,4-benzoquinone as a yellow oil which is used without further purification (Note 6).
C. 2,5-Di-tert-butyl-3,5,6-trichloro-2-cyclohexene-1,4-dione. A 2-l. Erlenmeyer flask is charged with a solution of 96.6 g. (0.379 mole) of crude 3-chloro-2,5-di-tert-butyl-1,4-benzoquinone in 500 ml. of glacial acetic acid. The reaction flask is equipped with an efficient magnetic stirring bar, and chlorine gas is introduced (Note 7). The reaction is complete in 4-5 hours (Note 8), at which time the solution is flushed with nitrogen to expel excess chlorine. Approximately 1 l. of water is added, and the resulting mixture is extracted with 300 ml. of dichloromethane. The dichloromethane solution is washed three times with water, dried over anhydrous magnesium sulfate, filtered, and concentrated on a rotary evaporator, yielding 116–117 g. (94%) of 2,5-di-tert-butyl-3,5,6-trichloro-2-cyclohexene-1,4-dione as a lemon yellow oil which is used directly in the next step (Note 9).
D. 2,5-Di-tert-butyl-3,6-dichloro-1,4-benezoquinone. A 2-l. Erlenmeyer flask is charged with a solution of 116.6 g. (0.3582 mole) of 2,5-di-tert-butyl-3,5,6-trichloro-2-cyclohexene-1,4-dione in 800 ml. of ether. To the vigorously swirled solution is added, in one portion, 26.2 g. (0.359 mole) of diethylamine dissolved in ca. 50 ml. of ether. The reaction,which is instantaneous, results in a voluminous precipitate (Note 10). The reaction mixture is washed with two 1-l. portions of water and then with 500 ml. of aqueous saturated sodium chloride (Note 11). The ether solution is dried over anhydrous magnesium sulfate, filtered, and concentrated on a rotary evaporator. The crude product, a yellow semisolid (109 g.), is dissolved in 300 ml. of hot ethanol, then cooled first to room temperature and finally to 0°. After crystallization has begun, the flask is left at −5° to −10° overnight. The product is filtered, washed with 85% ethanol, and air dried, yielding 62–70 g. (60–67%) of yellow crystalline 2,5-di-tert-butyl-3,6-dichloro-1,4-benzoquinone, m.p. 68–69° (Note 12) and (Note 13).
E. 3,6-Diazoido-2,5-di-tert-butyl-1,4-benzoquinone. A solution of 10 g. (0.035 mole) of 2,5-di-tert-butyl-3,6-dichloro-1,4-benzoquinone in 375 ml. of methanol is cooled to 5–15°. To the solution is added, over 1–2 minutes, a solution of 5 g. (0.08 mole) of sodium azide in 15 ml. of water. The initial yellow solution becomes orange during addition of the azide. The flask is then cooled to −5° to −10° for at least 4 hours. The product precipitates from the solution and is collected by filtration, yielding 8.3–8.8 g. (80–85%) of 3,6-diazido-2,5-di-tert-butyl-1,4-benzoquinone, m.p. 88.9–90° (dec.), which is recrystallized at room temperature by dissolving it in a minimum amount of chloroform, filtering, and adding 2 parts of 95% ethanol to the chloroform solution. The resulting solution is cooled to −5° to −10°; the crystalline precipitate is isolated (86% recovery) by filtration (Note 14), with no appreciable change in melting point (Note 15).
F. tert-Butylcyanoketene. Typically, the ketene is prepared by dissolving 1 g. (0.003 mole) of 3,6-diazido-2,5-di-tert-butyl-1,4-benzoquinone in 10–25 ml. of anhydrous benzene (Note 16). The solution is refluxed, and the disappearance of the starting material as well as the intermediate cyclopentenedione is followed by TLC (Note 17). When the cyclopentenedione is no longer detectable, after approximately 90 minutes, the heating is stopped. The solution contains tert-butylcyanoketene in amounts equivalent to at least a 95% yield (Note 18).
2. Notes
1.
Practical grade 2,5-di-tert-butyl-1,4-benzoquinone of m.p. 151–154° obtained from Eastman Organic Chemicals was used.
Chlorine available from Air Products and Chemicals, Inc., was used by the checkers.
2.
A satisfactory way to introduce
chlorine with minimal loss of the gas is to seal the reaction flask with a
two-holed stopper equipped with a gas-inlet tube, reaching just above the surface of the reaction mixture, and an exit tube, connected to a U-tube filled with mineral oil which is used as a gas-flow indicator.
Chlorine is introduced from the
cylinder through a safety trap at such a rate as to maintain a small positive pressure in the reaction flask.
3.
The reaction can be followed by
1H NMR spectroscopy. The original absorption for the vinyl proton disappears and two new absorption peaks appear, one in the vinyl region (
ca. δ 6.5, CDCl
3) and the other in the methine region of the spectrum. There are two products formed, presumably the
cis- and
trans-isomers, in the ratio of 95:5, respectively. The checkers also obtained the same yield when the reaction quantities were doubled.
4.
An analytical sample has a m.p. of
127–129°. Additional product can be recovered from the mother liquor by addition of approximately 1 l. of water followed by filtration. The yield of this product is about
31 g. (
21%). However, it contains about 20% of the minor isomer
(Note 3) that is not dehydrohalogenated under the reaction conditions employed in the next step. The second crop can be recrystallized from hot
methanol, giving predominantly the desired isomer. In some preparations the submitters did not separate the minor product and observed no significant loss in yield in the subsequent steps. The spectral properties of the product are as follows; IR (Nujol) cm.
−1; 1700 (C=O), 1600 (C=C);
1H NMR (CDCl
3), δ (multiplicity, number of protons, assignment): 1.28 [s, 9H, C(C
H3)
3], 1.37 (s, 9H, C(C
H3)
3], 4.75 (s, 1H, C
H), 6.47 (s, 1H, =C
H).
5.
The reaction mixture warms slightly, resulting in the boiling of the
ether. The large amount of
diethylamine hydrochloride formed transforms the reaction mixture into a thick paste.
6.
The spectral properties of the product are as follows; IR (Nujol) cm.
−1: 1680 (C=O), 1660 (C=C);
1H NMR (CDCl
3), δ (multiplicity, number of protons, assignment): 1.30 [s, 9H, C(C
H3)
3], 1.46 [s, 9H, C(C
H3)
3], 6.59 (s, 1H, =C
H).
7.
The experimental setup in this reaction is exactly as that described in
(Note 2).
8.
The progress of the reaction is followed by
1H NMR spectroscopy. When the absorption for the vinyl proton (
ca. δ 6.6, CDCl
3) is completely absent, the reaction is stopped. Several minor products that were not identified are also formed in this step.
9.
IR (Nujol) cm.
−1: 1710 (C=O);
1H NMR (CDCl
3), δ (multiplicity, number of protons, assignment): 1.1–1.4 [m, 18H, 2C(C
H3)
3], 4.68 (s, 1H, C
H), 4.87 (s, 1H, C
H).
10.
Comments given in
(Note 5) apply here also.
11.
The solution may have a brown tint, partially masking the yellow color of the quinone. The dark color is probably due to reaction of the
diethylamine with the
2,5-di-tert-butyl-3,6-dichloro-1,4-benzoquinone.
12.
The mother liquor and wash solution are combined and concentrated to 200 ml. on a rotary evaporator. Upon cooling, a second crop (
15–18 g.) of product is obtained. This second crop was a semisolid material. The spectral properties of the crystalline product are as follows; IR (Nujol) cm.
−1: 1660 (C=O);
1H NMR (CDCl
3), δ (multiplicity, assignment): 1.45 [s, C(C
H3)
3].
13.
Theoretically there remains about
22% of product to be isolated. Some of this material can be recovered indirectly by converting it to the diazide. The submitters diluted the mother liquor, which contains at most
23 g. (0.079 mole) of 2,5-di-tert-butyl-3,6-dichloro-1,4-benzoquinone, with
500 ml. of methanol, and then added, with swirling, a solution of
10.4 g. (0.0160 mole) of sodium azide in 30 ml. of water over a 2-minute period, turning, the yellow solution orange. It is cooled to −5° to −10°, and the resulting orange precipitate is collected, yielding
12 g. of the diazide. The minimum yield is thus
88%.
14.
Water can be added to the mother liquor, and the mixture extracted with
chloroform to increase the diazide yield to
95–98%. During the course of any purification method that might be employed the diazide should not be heated above 50° since decomposition occurs quite noticeably at that temperature. It is best to store the pure product below −5° in the dark since it undergoes a facile photochemical rearrangement to the
cyclopentenedione.
15.
IR (Nujol) cm.
−1: 2110 (N
3), 1640 (C=O);
1H NMR (CDCl
3), δ (multiplicity, assignment): 1.31 [s, C(C
H3)
3].
16.
The Discussion contains comments on the stability of
tert-butylcyanoketene in various solvents.
17.
TLC is carried out on silica gel using 1:1 (v/v)
petroleum ether–chloroform as eluent. The
cyclopentenedione has an
Rf value about half that of the diazide, and can be detected with an UV lamp when silica gel containing fluorescent indicator is used. The ketene undoubtedly reacts with the hydroxyl groups of the silica gel and remains at the origin. The checkers found the reaction to be complete in 1.5–2 hours. The yield was established by the checkers to be ≥
95% by
1H NMR, by integration studies in the presence of an internal standard.
18.
The submitters have not been successful in isolating
tert-butylcyanoketene by any method. If the solvent is removed, the ketene polymerizes. The spectral properties of the product are as follows; IR (C
6H
6) cm.
−1: 2220 (C≡N), 2130 (C=C=O);
1H NMR (C
6H
6), δ (multiplicity, assignment): 0.75 [s, C(C
H3)
3].
3. Discussion
In
benzene at room temperature,
tert-butylcyanoketene (TBCK) does not undergo rapid self-condensation;
2however, it is quite reactive toward cycloaddition reactions with alkenes, allenes, ketenes, imines, and formimidates.
3 The mechanisms of a number of these cycloaddition reactions have been investigated and in some cases have been shown to involve the formation of a zwitterionic intermediate. Typical examples of TBCK cycloadditions are summarized in the formula below.
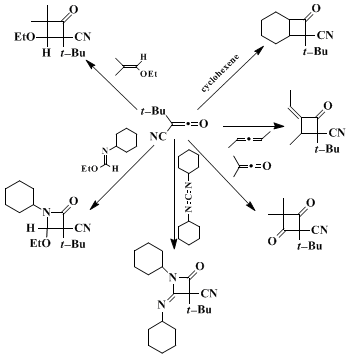
The ketene is less stable in nonaromatic hydrocarbon solvents than in aromatic solvents. For example, it has a half-life of greater than 7 days in
benzene at 25°. On the other hand, in
cyclohexane at the same temperature its half-life is only a few hours. All attempts to isolate
tert-butylcyanoketene have failed. Either removal of the solvent or cooling the solution to low temperature (−70°) causes polymerization of the ketene, a very efficient process, giving a white solid polymer which appears to have repeating ketenimine units. This assignment is consistent with the very strong absorption at 2140 cm
−1 in the IR spectrum.
4The method described here for the synthesis of tert-butylcyanoketene has marked advantages over other possible classical routes, e.g., dehydrohalogenation of the corresponding acid chloride. The only other product formed is molecular nitrogen and no external catalyst, e.g., triethylamine, is necessary. In fact, when tert-butylcyanoketene reacts with triethylamine, or when α-tert-butyl-α-cyanoacetyl chloride is subjected to dehydrohalogenation conditions, 1,3-di-tert-butyl-1,3-dicyanoallene is immediately formed and no ketene can be detected.
tert-Pentylcyanoketene can be prepared in an analogous fashion starting from the commercially available
2,5-di-tert-pentylbenzoquinone. This ketene seems to be very similar in stability and reactivity to the
tert-butyl homolog. Other cyanoketenes which have been prepared from azidoquinones or related compounds include dicyano-, chlorocyano-, bromocyano-, iodocyano-, methylcyano-, and
isopropylcyanoketene.
3 However, all of these must be generated
in situ since they undergo rapid self-condensation in the absence of a ketenophile.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
3,6-Diazoido-2,5-di-tert-butyl-1,4-benzoquinone
ethanol (64-17-5)
acetic acid (64-19-7)
Benzene (71-43-2)
methanol (67-56-1)
ether,
diethyl ether (60-29-7)
chloroform (67-66-3)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
cyclohexane (110-82-7)
chlorine (7782-50-5)
diethylamine (109-89-7)
sodium azide (26628-22-8)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
diethylamine hydrochloride (660-68-4)
triethylamine (121-44-8)
Butanenitrile, 2-carbonyl-3,3-dimethyl-,
tert-BUTYLCYANOKETENE (29342-22-1)
cyclopentenedione
isopropylcyanoketene
2,5-di-tert-butyl-1,4-benzoquinone
2,5-Di-tert-butyl-5,6-dichloro-2-cyclohexene-1,4-dione (33611-72-2)
3-Chloro-2,5-di-tert-butyl-1,4-benzoquinone (33611-70-0)
2,5-Di-tert-butyl-3,5,6-trichloro-2-cyclohexene-1,4-dione (117257-58-6)
2,5-Di-tert-butyl-3,6-dichloro-1,4-benzoquinone,
2,5-Di-tert-butyl-3,6-dichloro-1,4-benezoquinone (33611-73-3)
3,6-diazido-2,5-di-tert-butyl-1,4-benzoquinone (29342-21-0)
α-tert-butyl-α-cyanoacetyl chloride
1,3-di-tert-butyl-1,3-dicyanoallene
tert-Pentylcyanoketene
2,5-di-tert-pentylbenzoquinone
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved