Org. Synth. 1979, 59, 132
DOI: 10.15227/orgsyn.059.0132
PHOTOCHEMICAL RING CONTRACTION OF 2-ETHOXYPYRROLIN-5-ONES TO CYCLOPROPANONE DERIVATIVES: tert-BUTYL N-(1-ETHOXYCYCLOPROPYL)CARBAMATE
[Carbamic acid, (1-ethoxycyclopropyl)-, 1,1-dimethylethyl ester]
Submitted by Geoffrey C. Crockett and Tad H. Koch
1.
Checked by S. Boettger and M. F. Semmelhack.
1. Procedure
Caution! The photochemical reaction in Part C should be carried out behind a light-absorbent cover or shield. Protective goggles should be worn to avoid exposure of the eyes to ultraviolet light.
A. Succinimide silver salt (Note 1). A 3-l., two-necked, round-bottomed flask equipped with a mechanical stirrer and a pressure-equalizing dropping funnel is charged with a solution of 28.9 g. (0.292 mole) of succinimide (Note 2) in 1.2 l. of absolute ethanol (Note 3). A solution of 48.58 g. (0.2860 mole) of silver nitrate in 200 ml. of dimethyl sulfoxide (Note 4) is added in one portion. The resulting solution is stirred as 700 ml. (0.280 mole) of 0.400 M sodium ethoxide in ethanol (Note 5) and (Note 6) is added dropwise over 1.5 hours. The off-white silver salt begins to precipitate after ca. 140 ml. of the sodium ethoxide solution has been added. Stirring is continued for 45 minutes after the addition is completed, and the reaction mixture is then stored in a refrigerator at ca. 5° overnight to complete the precipitation and aggregation of the product.
The precipitate is collected on filter paper (Note 7) in a Büchner funnel by vacuum filtration and washed with 100 ml. of absolute ethanol. The solid is slurried in three 75-ml. portions of distilled water (Note 8), 100 ml. of absolute ethanol, two 100-ml. portions of reagent grade acetone, and two 100-ml. portions of anhydrous ethyl ether. The filter cake is pressed dry in the funnel with suction using a piece of rubber dam, transferred to a tared, 500-ml., round-bottomed flask, and dried under reduced pressure (0.01 mm.) at room temperature for 24 hours (Note 9), yielding 51–54 g. (88–94%) of the silver salt of succinimide.
B. 2-Ethoxypyrrolin-5-one. The flask containing 51–54 g. (0.25–0.26 mole) of succinimide silver salt is equipped with a magnetic stirring bar (Note 10), a heating mantle, and reflux condenser bearing a silica gel drying tube. The solid is suspended in 295 ml. of dry chloroform (Note 11), and 51.4 g. (26.4 ml., 0.330 mole) of ethyl iodide is added in one portion. The flask is covered with aluminum foil, and the mixture is stirred vigorously and heated under reflux for 48 hours. The mixture is cooled, the silver iodide is removed by vacuum filtration through Celite, and the filter cake is washed well with dry chloroform. The filtrate is concentrated by rotary evaporation to a mixture of a dark liquid and a white solid identified as succinimide. Anhydrous diethyl ether is added to dissolve the liquid, and the resulting suspension is filtered through a plug of glass wool, separating 7.7–11.5 g. of succinimide. The ether is removed from the filtrate by rotary evaporation at aspirator pressure, and the residual liquid is distilled under reduced pressure with a short-path distillation apparatus. The product is collected at 74–82° (0.01 mm.) as a faintly yellow oil which crystallizes in the freezer. The yield is 11.5–16.7 g. (32–46% based on sodium ethoxide in Part A) (Note 12) and (Note 13).
C. tert-Butyl N-(1-ethoxycyclopropyl)carbamate. A three-necked, cylindrical irradiation vessel is equipped with a magnetic stirring bar, a water-jacketed quartz immersion well, an inert gas-inlet, and a gas-exit tube connected to a bubbler (Note 14). The vessel is charged with 6.26 g. (0.0493 mole) of redistilled 2-ethoxypyrrolin-5-one (Note 20) and 180 ml. of dry tert-butyl alcohol (Note 15). The solution is stirred and degassed by bubbling nitrogen or argon through the gas-inlet tube for 15 minutes. The degassed solution is stirred and irradiated with ultraviolet light from a 450-watt, Hanovia, medium-pressure, mercury lamp filtered through a Vycor glass sleeve. During the irradiation an atmosphere of nitrogen or argon is maintained, and the lamp is cooled with warm water (35–40°) circulated through the cooling jacket of the immersion well. The progress of the irradiation is monitored by GC (Note 16). When 90% of the 2-ethoxypyrrolin-5-one has reacted, the irradiation is stopped. The solution (Note 17) is transferred to a 250-ml., round-bottomed flask equipped with a magnetic stirring bar and an air-cooled reflux condenser mounted with a T-shaped nitrogen inlet. Nitrogen is passed through the apparatus for 30 minutes, after which the solution is stirred and heated at reflux under a nitrogen atmosphere for 20 hours (Note 18). The solvent is removed by rotary evaporation, and the residual orange oil is refrigerated to induce crystallization. Sublimation of the solid at 35–40° (0.05 mm.) affords 5.5–6.3 g. (56–64%) of the carbamate as white needles, m.p. 38–40° (Note 19) and (Note 20).
2. Notes
1.
In parts A and B care should be taken to minimize the exposure of silver-containing reactants and products to light.
2.
Succinimide purchased from MC and B Manufacturing Chemists was used without purification.
3.
Absolute
ethanol from a commercial supplier was used.
4.
A mixture of
silver nitrate and
dimethyl sulfoxide was stirred vigorously for
ca. 1 hour to dissolve all of the salt.
Reagent grade dimethyl sulfoxide was used without purification.
5.
The
sodium ethoxide solution was prepared from the reaction of
9.2 g. (0.40 mole) of sodium with
1 l. of absolute ethanol and is standardized by titration with aqueous
0.1 N hydrochloric acid. The appropriate volume of the solution to give 0.280 mole of base was used.
6.
Slightly less than equivalent amounts of both
silver nitrate and
sodium ethoxide are used to minimize the formation of
silver oxide which imparts a brown color to the product.
7.
The submitters state that the use of a
sintered-glass funnel may cause discoloration of the product. However, the checkers used a sintered glass funnel in one run with no adverse effect on the yield or purity of the product.
8.
A considerable amount of
sodium nitrate is present in the precipitate. Although the presence of
sodium nitrate did not hinder small-scale alkylation reactions (
ca. 250 mg.), the submitters recommend that it be removed in larger runs to facilitate the isolation and drying of the silver salt.
These washings are most easily done without removing the material from the filter. However, the solid must be slurried thoroughly in each portion of solvent, particularly with acetone and ether. Care must be taken to ensure that the filter paper is not lifted from the bottom of the funnel. The submitters accomplished this by holding the filter paper in the funnel with a ring of flexible polyvinyl chloride (inside diameter 0.64 cm.), the ends of which were joined by a small piece of rigid polyethylene tubing. The ring was expanded to fit snugly in the bottom of the funnel over the paper.
9.
Silver salts may be unstable when heated. An explosion occurred while the
silver salt of isatin was drying under reduced pressure at
ca. 100°.
10.
The checkers found that the heavy suspension could not be stirred effectively with a magnetic stirring bar and recommend that a mechanical stirrer be used.
11.
Reagent grade chloroform was dried by filtering through alumina (50 g. per l. of solvent).
12.
The checkers' data are given. The submitters recovered
4.7–11.4 g. of succinimide and collected
15.6–21.2 g. (
44–60% based on
sodium ethoxide in Part A) of product, b.p.
65–70° (0.05 mm.). Based on the amount of unrecovered
succinimide, the yield of product obtained by the checkers and submitters was
49–61% and
68–69%, respectively. The product is best stored in a freezer. If sufficient care is taken to exclude moisture,
2-ethoxypyrrolin-5-one is stable indefinitely.
13.
The product obtained by the submitters was contaminated with impurities amounting to
ca. 10% which were primarily
succinimide and
N-ethylsuccinimide. Although this material was considered to be of satisfactory purity for use in Part C, further purification can be accomplished, if desired, by redistillation, giving product boiling at
72–74° (0.05 mm.). The checkers found it necessary to redistil the
2-ethoxypyrrolin-5-one to obtain product with the reported melting point in Part C
(Note 20); IR (neat) cm.
−1: 2940, 1748, 1562;
1H NMR (CDCl
3), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 1.4 (t,
J = 7, 3H, OCH
2C
H3), 2.4–3.0 (m, 4H, C
H2C
H2), 4.45 (q,
J = 7, 2H, OC
H2CH
3); UV (cyclohexane) nm. max. (ε): 273 (55).
14.
The irradiation apparatus was similar to one depicted in the procedure for
bicyclo[2.1.0]pent-2-ene in
Org. Synth., Coll. Vol. 6, 145 (1988), Figure 2, Section A. The height and inside diameter of the irradiation vessel used by the submitters were approximately 35 cm. and 6.2 cm., respectively. Two short necks with
T 14/20 outer joints were located on the shoulder of the vessel just below the
T 60/50 center joint. One neck was capped with a
rubber septum and the other was connected to the exit bubbler. The nitrogen inlet was a syringe needle passing through the septum and connected to a section of Teflon tubing that extended to the bottom of the vessel. The checkers used a similar
23 × 7.5 cm. irradiation vessel that had a
fritted-glass inlet for
argon situated at the base as shown in the figure referred to above. The solution was agitated during the irradiation by a continual flow of
argon rather than by magnetic stirring.
The apparatus is dried in an oven at 140° overnight and cooled under nitrogen or argon prior to the irradiation. A Vycor filter sleeve and a 450-watt, medium-pressure mercury lamp are placed in the immersion well. The Vycor filter, the quartz immersion well (catalog No. 19434), the 450-watt mercury lamp (catalog No. 679A36), and the requisite transformer are all available from Hanovia Lamp Division, Canrad-Hanovia Inc., 100 Chestnut Street, Newark, New Jersey 07105.
15.
Reagent grade tert-butyl alcohol was distilled from
calcium hydride prior to use. The scale described is that used by the checkers. The submitters irradiated
4.0 g. (0.032 mole) of 2-ethoxypyrrolin-5-one in 115 ml. of dry tert-butyl alcohol.
16.
The submitters used a
2.1 m. × 0.64 cm. column with 5% fluorosilicone (FS-1265) supported on Diatoport S as stationary phase. With a column temperature of 170° and a
helium flow rate of 60 ml. per minute,
2-ethoxypyrrolin-5-one has a retention time of 2.2 minutes. The analysis was carried out at 160° by the checkers, using a column of
5% diethylene glycol succinate–Bentone34 supported on Diatoport S. The starting material had a retention time of 3.9 minutes under these conditions.
Bentone34 is available from Applied Sciences Laboratory, Box 440, State College, Pennsylvania 16801.
17.
At this point the product consisted mostly of the isocyanate, since the reaction with
tert-butyl alcohol is relatively slow at 35–40°. If the photolysis is carried out in an aprotic solvent such as
tetrahydrofuran, the isocyanate may be isolated.
2 However, care must be exercised to avoid losses of this rather volatile and moisture-sensitive compound.
18.
The isocyanate is completely consumed at this time, as evidenced by the disappearance of the absorption band at 2250 cm.
−1 in the IR spectrum.
19.
Alternatively, the product may be distilled at
40° (0.05 mm.). However, the distillate tends to crystallize in the condenser and plug the apparatus.
20.
The checkers found that product with the reported melting point was obtained only when the starting
2-ethoxypyrrolin-5-one was redistilled carefully and was largely free of the
N-ethyl isomer and
succinimide. With
2-ethoxypyrrolin-5-one purified by a single distillation, the product was obtained as a gummy solid that was difficult to purify. Nevertheless, the IR and
1H NMR spectra of this material were essentially identical to those of pure
tert-butyl N-(1-ethoxycyclopropyl)carbamate. The submitters obtained
4.4–4.8 g. (
70–76%) of carbamate, m.p.
40–42°, from
4.0 g. of 2-ethoxypyrrolin-5-one. The product has the following spectral characteristics: IR (neat) cm.
−1: 3333, 2940, 1754 (C=O);
1H NMR (CDCl
3), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 0.80–1.15 (m, 4H, cyclopropyl
H), 1.13 (t,
J = 7, 3H, OCH
2C
H3), 1.47 [s, 9H, C(C
H3)
3], 3.68 (q,
J = 7, 2H, OC
H2CH
3), 5.75 (broad, 1H, N
H).
3. Discussion
The procedure described here for the preparation of
succinimide silver salt is a modification of one reported for the formation of the silver derivative of maleimide.
3 The alkylation step is modeled after the procedure of Comstock and Wheeler,
4 who prepared
2-ethoxypyrrolin-5-one in unspecified yield, and is an improvement over a later procedure developed in the laboratories of the submitters.
2 The general scheme has been successfully applied to the preparation of a variety of 2-ethoxypyrrolin-5-ones (Table I)
5,6,7 as well as
6-ethoxy- and 6-propoxy-4,5-dihydro-2(3H)-pyridone from the corresponding five- and six-membered cyclic imides.
2The photochemical rearrangement of substituted 2-ethoxypyrrolin-5-ones is a general reaction of synthetic utility and high stereoselectivity, which affords the corresponding 1-ethoxycyclopropyl isocyanates and their derivatives in useful yields (Table I).
6,7 The procedure reported here is the only known preparation of
tert-butyl N-(1-ethoxycyclopropyl)carbamate, a precursor of
1-aminocyclopropanol and
1-ethoxycyclopropylamine.
8 1-Aminocyclopropanol has previously been prepared in low yield by the addition of
ammonia to
cyclopropanone.
8 The photorearrangement of
2-ethoxypyrrolin-5-one to
tert-butyl N-(1-ethoxycyclopropyl)carbamate followed by hydrolysis to
1-aminocyclopropanol is a key step in the synthesis of the alkaloid coprine.
8 Cyclopropanone derivatives have been used as precursors for a variety of compounds
9 such as β-lactams,
10 cyclobutanones,
11 and cyclopropanols.
12
TABLE I
PREPARATION AND IRRADIATION OF 2-ETHOXYPYRROLIN-5-ONES
|
|
2-Ethoxypyrrolin-5-one
|
Yield (%)
|
Photoproduct or Its Derivative
|
Yield (%)
|
|
|
|
46a
|
|
43b
|
|
|
59
|
|
76b
|
|
|
89
|
|
64b
|
|
|
74
|
|
53b
|
|
|
88c
|
|
58d
|
|
|
60
|
|
aThe yield was 84% based on unrecovered imide.
|
bThe product is a mixture of endo- and exo- isomers.
|
cThe isomers were separated by preparative GC.
|
dThe product is a mixture of cis- and trans- isomers.
|
2-Ethoxypyrrolin-5-one reacts with secondary amines, giving 2-aminopyrrolin-5-ones which photochemically rearrange to aminocyclopropyl isocyanates in 80–90% yield.
13 Furthermore,
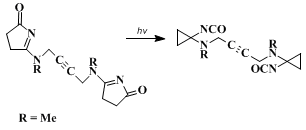
1,4-bis[(pyrrolin-3-onyl)methylamino]-2-butyne photorearranges to
1,4-bis[(isocyanatocyclopropyl)methylamino]-2-butyne. This reaction is of potential use for photocrosslinking polyurethanes and polyureas.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
6-ethoxy- and 6-propoxy-4,5-dihydro-2(3H)-pyridone
Bentone34
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
ammonia (7664-41-7)
ether,
ethyl ether,
diethyl ether (60-29-7)
chloroform (67-66-3)
silver oxide (20667-12-3)
silver nitrate (7761-88-8)
nitrogen (7727-37-9)
aluminum (7429-90-5)
acetone (67-64-1)
sodium (13966-32-0)
sodium ethoxide (141-52-6)
sodium nitrate
Succinimide (123-56-8)
Ethyl iodide (75-03-6)
Tetrahydrofuran (109-99-9)
dimethyl sulfoxide (67-68-5)
argon (7440-37-1)
tert-butyl alcohol (75-65-0)
silver iodide (7783-96-2)
calcium hydride (7789-78-8)
helium (7440-59-7)
diethylene glycol succinate
Bicyclo[2.1.0]pent-2-ene (5164-35-2)
CYCLOPROPANONE (5009-27-8)
Carbamic acid, (1-ethoxycyclopropyl)-, 1,1-dimethylethyl ester,
tert-Butyl N-(1-ethoxycyclopropyl)carbamate (28750-48-3)
Succinimide silver salt,
silver salt of succinimide
2-Ethoxypyrrolin-5-one (29473-56-1)
1-aminocyclopropanol
1-ethoxycyclopropylamine
1,4-bis[(pyrrolin-3-onyl)methylamino]-2-butyne
1,4-bis[(isocyanatocyclopropyl)methylamino]-2-butyne
N-ethylsuccinimide (2314-78-5)
silver salt of isatin
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved