Org. Synth. 1970, 50, 15
DOI: 10.15227/orgsyn.050.0015
m-CHLOROPERBENZOIC ACID
[Benzenecarboperoxoic acid, 3-chloro-]
Submitted by Richard N. McDonald
1, Richard N. Steppel, and James E. Dorsey.
Checked by William N. Washburn and Ronald Breslow.
1. Procedure
Caution! Reactions and subsequent operations involving peracids and peroxy compounds should be run behind a safety shield. For relatively fast reactions, the rate of addition of the peroxy compound should be slow enough so that it reacts rapidly and no significant unreacted excess is allowed to build up. The reaction mixture should be stirred efficiently while the peroxy compound is being added, and cooling should generally be provided since many reactions of peroxy compounds are exothermic. New or unfamiliar reactions, particularly those run at elevated temperatures, should be run first on a small scale. Reaction products should never be recovered from the final reaction mixture by distillation until all residual active oxygen compounds (including unreacted peroxy compounds) have been destroyed. Decomposition of active oxygen compounds may be accomplished by the procedure described in Korach, M.; Nielsen, D. R.; Rideout, W. H. Org. Synth. 1962, 42, 50 (Org. Synth. 1973, Coll. Vol. 5, 414). [Note added January 2011].
A 1-l. beaker (Note 1) equipped with a magnetic stirrer is charged with 1.5 g. of magnesium sulfate heptahydrate, 36 g. of sodium hydroxide, 360 ml. of water, 90 ml. of 30% hydrogen peroxide, and 450 ml. of dioxane. This mixture is cooled to 15° with an ice-water bath and by the addition of a small amount of ice to the mixture before 52.5 g. (0.300 mole) of m-chlorobenzoyl chloride (Note 2) is added in one portion while vigorous stirring is maintained. Small portions of ice are added to maintain the temperature below 25°. The reaction mixture is stirred at this temperature for 15 minutes, then transferred to a 3-l. separatory funnel. Cold, 20% sulfuric acid (900 ml.) (Note 3) is added to the separatory funnel, the mixture is shaken and separated, and the aqueous layer is extracted with four 200 ml. portions of cold dichloromethane (Note 4). The combined extracts are dried over anhydrous magnesium sulfate, and the dichloromethane is removed under reduced pressure via a short-path distillation (Note 5). After most of the solvent has been removed, a white pasty solid remains and the residual solvent is removed under full vacuum for an additional 2 hours or until a white, flaky powder remains. The product weighs approximately 51 g. Sodium thiosulfate analysis indicates 80–85% active oxygen present (Note 6).
2. Notes
1.
The checkers used a
Pyrex vessel; the submitters utilized a
Nalgene beaker, because contact with the glass surface may catalyze the decomposition of the peracid.
2.
The
m-chlorobenzoyl chloride is prepared by refluxing
m-chlorobenzoic acid, either commercial or recovered, with excess
thionyl chloride. Distillation gives the
m-chlorobenzoyl chloride in high yield, b.p.
112° (11 mm.).
2The recovery of the m-chlorobenzoic acid, the by-product from m-chloroperbenzoic acid oxidations, is facilitated by using dichloromethane as the solvent since the peracid is very soluble whereas the acid is quite insoluble.
3.
The cold
20% sulfuric acid solution is made by adding concentrated
sulfuric acid (180 ml.) to crushed ice followed by the addition of more ice until the required volume is reached,
i.e., 900 ml. The acid solution is kept cold in an
ice bath until required. The
dichloromethane is also precooled before use.
4.
Iodometric titration of the moist extracts indicates approximately
51 g. of
m-chloroperbenzoic acid present. A 4.0-ml. aliquot of the solution requires approximately
20 ml. of a 0.1000 N solution of sodium thiosulfate. To prepare the sample,
10 ml. of 10% sodium iodide and
5 ml. of acetic acid are added to the 5-ml. aliquot, and the mixture is diluted to 50 ml. The dark red solution is titrated to a pale yellow. At this point
1 ml. of starch solution is added and the titration continued to the end point,
i.e., a change from dark blue to clear.
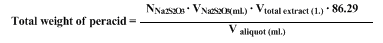
5.
The
dichloromethane solvent should be removed as rapidly as possible because contact with glass and heat cause decomposition of the peracid. A convenient method for the removal of the solvent involves a short-path vacuum distillation using a
2-l. distillation flask and trapping the solvent at dry ice or liquid
nitrogen temperatures. The pressure should be reduced slowly at first and at least three
traps used to minimize the amount of solvent introduced into the
vacuum pump. Adjustment of a stopcock located between the first and second traps will help to control this problem. The distillation flask is placed in a
water bath maintained between 25° and 35° so that the rate of solvent evaporation is quite rapid. Removal of the solvent over prolonged periods and drying of the solid peracid by excessive heating results in drastic losses of active
oxygen.
m-Chloroperbenzoic, once isolated, is stable over long periods of time when stored in
polyethylene containers and refrigerated.
6.
Iodometric titration of the solid product involves the use of 0.2-g. samples of the peracid and the procedure in
(Note 4).
3. Discussion
This method is an extension of the reported perhydrolysis of certain other acid chlorides and anhydrides.
3 Although
m-chloroperbenzoic acid is commercially available, this preparation requires only a short reaction time and simple equipment, and it affords high yields of this relatively stable and useful peracid. The
dichloromethane–
dioxane extracts can be stored and used directly for many peroxidations, in which case the total preparation time should not exceed 2 hours.
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
sulfuric acid (7664-93-9)
acetic acid (64-19-7)
sodium hydroxide (1310-73-2)
thionyl chloride (7719-09-7)
oxygen (7782-44-7)
sodium thiosulfate (7772-98-7)
nitrogen (7727-37-9)
hydrogen peroxide (7722-84-1)
sodium iodide (7681-82-5)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
dioxane (123-91-1)
magnesium sulfate heptahydrate (10034-99-8)
m-Chloroperbenzoic acid,
Benzenecarboperoxoic acid, 3-chloro- (937-14-4)
m-chlorobenzoic acid (535-80-8)
m-chlorobenzoyl chloride (618-46-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved