Org. Synth. 1972, 52, 96
DOI: 10.15227/orgsyn.052.0096
PREPARATION OF CYANO COMPOUNDS USING ALKYLALUMINUM INTERMEDIATES: 1-CYANO-6-METHOXY-3,4-DIHYDRONAPHTHALENE
[Naphthalenecarbonitrille, 3,4-dihydro-6-methoxy-]
Submitted by W. Nagata
1, M. Yoshioka, and M. Murakami.
Checked by R. Wong, C. Kowalski, R. Czarny, and R. E. Ireland.
1. Procedure
Caution! Benzene has been identified as a carcinogen; OSHA has issued emergency standards on its use. All procedures involving benzene should be carried out in a well-ventilated hood, and glove protection is required.
A
200-ml., two-necked round-bottomed flask charged with
6.15 g. (0.0347 mole) of 6-methoxy-1-tetralone (Note 1) and a
100 ml., round-bottomed flask are flushed with
nitrogen, and each of the flasks is fitted with an adaptor with a side arm connected to a
nitrogen bubbler system and then charged with
30 ml. of anhydrous toluene. The
200-ml. flask is cooled to −20° to −25° (bath temperature)
(Note 2). Into the
100-ml. flask is introduced
60 ml. (0.07 mole) of a 13% solution of diethylaluminum cyanide in
benzene (Note 3) with a hypodermic syringe, and this flask is cooled with ice water. The cooled
diethylaluminum cyanide solution is added to the cold solution of
6-methoxytetralone with a hypodermic syringe and the resulting mixture, after being swirled, is kept at −15° for 80 minutes under
nitrogen. The stopper of the flask is replaced by a glass tube which has one end extending to the bottom of the reaction flask and the other end mounted in a neck of a
2-l., three-necked flask, equipped with an
efficient stirrer and containning a cold (−70°) mixture of
250 ml. of methanol and
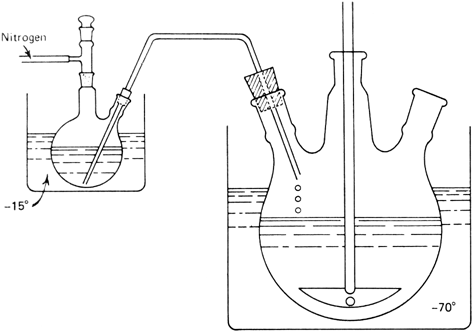
150 ml. of concentrated hydrochloric acid as shown in
Figure 1. The reaction mixture is added through the glass tube to the vigorously stirred acid mixture by applying a positive
nitrogen pressure to the reaction flask
(Note 4). After the bulk of the reaction mixture is added, about
50 ml. of a cold mixture of methanol and
hydrochloric acid is added to the reaction flask and this mixture is transferred to the
2-l. flask in the same way, as described above. Stirring is continued for one hour, and the resulting mixture is poured into a mixture of
200 ml. of concentrated hydrochloric acid and 1 l. of ice water
(Note 5) and extracted with three
500-ml. portions of dichloromethane. The combined organic phases are washed once with 1.5 l. of water, dried over anhydrous
sodium sulfate, and evaporated from a flask containing
55 mg. of
p-toluenesulfonic acid monohydrate (Note 6). using a
rotary evaporator at a temperature below 40°.
Figure 1. Apparatus for acid treatment of the reaction mixture.
The residue, obtained as a pale yellow oil, weighs approximately 7.4 g. and consists of 1-cyano-1-hydroxy-6-methoxytetralin and a small amount of unchanged 6-methoxy-1-tetralone. The oil is transferred to a 10-ml. Claisen flask, a small amount of a mixture of dichloromethane and diethyl ether being used to complete the transfer. Two hundred milligrams of powdered potassium hydrogen sulfate is added, and the flask is heated at 130° under reduced pressure (5 mm.) for 30 minutes. The pressure is then reduced to 0.01 mm. and the temperature is raised to about 150°, collecting all the distillate [b.p. 113–117° (0.01 mm.)] in a 50-ml. flask. The viscous distillate (including material adhering to the distillation apparatus), weighs 6.0–6.2 g. and yields 4.91–5.05 g. (76–78%) of product, m.p. 50–51.5°, after two or three crystallizations from methanol. The residue from the mother liquors (1.0–1.3 g.) is adsorbed on a column of 100 times its weight of silica gel (70–325 mesh), and the column is eluted with approximately 1 l. of 40% ether in petroleum ether (b.p. 30–60°). The first 200 ml. of eluent is discarded, and 510–550 mg. of the product is eluted in the next 250 ml. of eluent. Crystallization of this material from an ether–petroleum ether (b.p. 30–60°) mixture affords an additional 460–500 mg. (7.0–7.8%) of pure product, m.p. 50.5–51.5°. The total yield of the unsaturated nitrile is 5.41–5.51 g. (83.8–85.5%). (Note 7). The final 500 ml. fraction from chromatography contains 330–660 mg. (5.4–10.7%) of the starting material, m.p. 77–78°.
2. Notes
1.
6-Methoxy-1-tetralone is available from K & K Laboratories, New York, although the submitters used a material, m.p.
77–80°, produced by Osaka Yuki Gosei K. K., Nishinomiya-shi, Japan.
2.
Crystals of
6-metoxy-1-tetralone may separate from the solution on cooling, but redissolve upon addition of the cooled
diethylaluminum cyanide solution.
3.
For the preparation of
diethylaluminum cyanide, see
Org. Synth., Coll. Vol. 6, 436 (1988). Both the submitters and the checkers employed a crude reagent solution rather than a solution prepared from distilled
diethylaluminum cyanide. A 1–2
M solution of
diethylaluminum cyanide in
benzene is commercially available from Alfa Products, Ventron Corporation, Danvers, Massachusetts.
4.
Application of the
nitrogen pressure may be made conveniently by capping the outlet of the mercury bubbler.
5.
The two-step decomposition is effective for preventing reconversion of the cyanohydrin into the starting ketone.
6.
The cyanohydrin initially formed is unstable and readily reconverted to the starting
6-methoxy-1-tetralone on evaporation of the extracts unless the solution is kept slightly acidic by addition of a trace amount of
p-toluenesulfonic acid monohydrate. As this acid is relatively insoluble in
dichloromethane, it should be added directly to the flask used for evaporation of the solvent.
7.
Preferably, the product should be stored in an oxygen-free atmosphere. Samples not stored in an inert atmosphere have deteriorated to dark-brown masses within several months, whereas no appreciable change has been observed in a sample stored for 2 years in an ampoule filled with
argon.
3. Discussion
The present method developed by the submitters
2 is the only practical process for the preparation of
1-cyano-6-methoxy-3,4-dihydronaphthalene. Birch and Robinson
3 have reported that
6-methoxy-1-tetralone did not react with
hydrogen cyanide or
sodium acetylide.
This process presents a procedure applicable to the preparation of cyanohydrins from relatively unreactive ketones and aldehydes. 1-Cyano-6-methoxy-3,4-dihydronaphthalene is useful as an intermediate in the synthesis of polycyclic compounds.
Cyanotrimethylsilane4 is useful for the preparation of trimethylsilyl ethers of cyanohydrins, obtained from ketones, aldehydes, α,β-unsaturated ketones, and quinones.
5
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
petroleum ether
Naphthalenecarbonitrille, 3,4-dihydro-6-methoxy-
6-metoxy-1-tetralone
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
methanol (67-56-1)
ether,
diethyl ether (60-29-7)
hydrogen cyanide (74-90-8)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
potassium hydrogen sulfate (7646-93-7)
toluene (108-88-3)
dichloromethane (75-09-2)
sodium acetylide
argon (7440-37-1)
Cyanotrimethylsilane (7677-24-9)
6-methoxy-1-tetralone,
6-methoxytetralone (1078-19-9)
Diethylaluminum cyanide (5804-85-3)
1-cyano-1-hydroxy-6-methoxytetralin
1-Cyano-6-methoxy-3,4-dihydronaphthalene (6398-50-1)
p-toluenesulfonic acid monohydrate (6192-52-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved