Org. Synth. 1973, 53, 107
DOI: 10.15227/orgsyn.053.0107
REDUCTION OF ALKYL HALIDES AND TOSYLATES WITH SODIUM CYANOBOROHYDRIDE IN HEXAMETHYLPHOSPHORIC TRIAMIDE (HMPA):
A. 1-IODODECANE TO n-DECANE
B. 1-DODECYL TOSYLATE TO n-DODECANE
Submitted by Robert O. Hutchins
1, Cynthia A. Milewski, and Bruce E. Maryanoff.
Checked by Ronald I. Trust and Robert E. Ireland.
1. Procedure
Caution!
Hexamethylphosphoric triamide (HMPA) vapors have been reported to cause cancer in rats.
2 All operations with
hexamethylphosphoric triamide should be performed in a good
hood, and care should be taken to keep the liquid off the skin.
A.
n-Decane. A dry,
100-ml., three-necked flask equipped with a
stirring bar, a
thermometer, and a
condenser protected with a
drying tube is charged with
25 ml. of hexamethylphosphoric triamide (HMPA) (Note 1),
1-iododecane (2.7 g., 0.010 mole) (Note 2) and
sodium cyanoborohydride (0.943 g., 0.0157 mole) (Note 3). The solution is stirred at 70° for 2 hours, diluted with 25 ml. of water, and extracted with three
30-ml. portions of diethyl ether. The combined extracts are washed twice with water and dried over anhydrous
magnesium sulfate. The solvent is removed by distillation on a
steam bath through a
12-in. vacuum-jacketed Vigreux column (Note 4). The residue is distilled at reduced pressure in a short-path apparatus
(Caution! foaming), yielding
1.25–1.29 g. (
88–90%)
(Note 5),
(Note 6) of
n-decane, b.p.
68–70° (14 mm.);
n20D 1.4122,
n26D 1.4085 (lit.,
3 n25D 1.4097
(Note 7).
B.
n-Dodecane. A dry,
200-ml., three-necked flask equipped exactly as described in Section A is charged with
50 ml. of hexamethylphosphoric triamide (HMPA),
1-dodecyl tosylate (6.80 g., 0.0201 mole) (Note 8), and
sodium cyanoborohydride (5.02 g., 0.0797 mole) (Note 3). The solution is stirred at 80° for 12 hours
(Note 9), diluted with 50 ml. of water, and extracted with three
60-ml. portions of hexane. The
hexane solution is washed twice with water, dried over anhydrous
magnesium sulfate, and concentrated with a
rotary evaporator. Distillation of the residue through a short-path apparatus
(Note 5) (Caution! foaming) affords
2.49–2.64 g. (
73–78%) of
n-dodecane, b.p.
79–81°; (3.75 mm.) n24D 1.4217 (lit.,
4 n20D 1.4219)
(Note 7).
2. Notes
1.
Commercial hexamethylphosphoric triamide was distilled from
calcium hydride and stored over 13X molecular sieves (Linde).
2.
Commercial 1-iododecane (Eastman Organic Chemicals) was filtered through activated charcoal and distilled before use.
3.
Sodium cyanoborohydride was used as received from Aldrich Chemical Company, Inc., or Alfa Products, Thiokol/Ventron Division. No purification is necessary. Tan or brown material may be purified by the method of Purcell
5 or of Borch.
54.
If a
hexane workup is used, and the solvent is removed with a rotary evaporator, considerable loss of product results from codistillation with the
hexane. This should not present a significant problem when higher boiling materials are produced.
5.
The condenser was cooled with an
ethylene glycol–water mixture at −5°, and the receiver was cooled to −10° in an
ice–salt bath.
6.
Considerable mechanical loss was observed because of inability to distill the last portions of the product at 14 mm. To avoid this problem, the pressure was reduced near the end of the distillation to
ca. 5 mm. This did not affect the purity of the product (
(Note 7)).
7.
Both products showed IR and
1H NMR spectra identical to those of authentic samples, and no side products were detected by GC or
1H NMR.
8.
Dodecyl tosylate was prepared from
1-dodecanol by the procedure in
Org. Synth., Coll. Vol. 3, 366 (1955). Crystallization from a dried (
magnesium sulfate) solution in light
petroleum ether afforded white needles, m.p.
27.5–28.5°.
9.
The large excess of
sodium cyanoborohydride is recommended for the reduction of tosylates. Use of reduced molar excesses led to substantially lower yields. For example, a 3:1
cyanoborohydride to
tosylate ratio afforded less than
60% yield of product at 80° for 5 hours, while a 1.5:1 excess gave only
52% yield at 70° for 8 hours.
3. Discussion
These preparations illustrate the use of
sodium cyanoborohydride in
hexamethylphosphoric triamide as an effective, selective, and convenient procedure for the reduction of alkyl halides and tosylates, and are essentially the same as previously described.
6 The very mild reducing ability of
sodium cyanoborohydride makes the method particularly valuable when other functional groups are present in the molecule. In addition, alkene side-products are seldom encountered, contrary to the situation with
lithium aluminum hydride7 or
sodium borohydride in aqueous diglyme.
8 The combination of
sodium borohydride in polar aprotic solvents is also effective for halide and
tosylate removal,
9 although it is less selective.
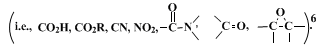
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
petroleum ether
diethyl ether (60-29-7)
1-dodecanol (112-53-8)
ethylene glycol (107-21-1)
magnesium sulfate (7487-88-9)
lithium aluminum hydride (16853-85-3)
hexane (110-54-3)
calcium hydride (7789-78-8)
sodium borohydride (16940-66-2)
n-DECANE (124-18-5)
hexamethylphosphoric triamide (680-31-9)
tosylate (104-15-4)
sodium cyanoborohydride (25895-60-7)
cyanoborohydride
1-IODODECANE (2050-77-3)
1-DODECYL TOSYLATE,
Dodecyl tosylate (10157-76-3)
n-DODECANE (112-40-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved