Org. Synth. 1976, 55, 57
DOI: 10.15227/orgsyn.055.0057
FREE-RADICAL CYCLIZATION: ETHYL 1-CYANO-2-METHYLCYCLOHEXANECARBOXYLATE
[Cyclohexanecarboxylic acid, 1-cyano-2-methyl-, ethyl ester]
Submitted by Marc Julia and Michel Maumy
1.
Checked by Edward J. Zaiko and Herbert O. House.
1. Procedure
Caution! Since hydrogen is evolved in this procedure, it should be performed in an efficient hood.
A. Ethyl (E)-2-cyano-6-octenoate. A solution of 38.4 g. (0.384 mole) of (E)-4-hexen-1-ol (Note 1) in 160 ml. of anhydrous pyridine is placed in a 1-l. Erlenmeyer flask equipped with a magnetic stirring bar, and the solution is cooled in an ice bath. Over 1 hour, 91.5 g. (0.478 mole) of p-toluenesulfonyl chloride (Note 2) is added portionwise and with stirring to the reaction mixture while maintaining the temperature at 0–5°. The resulting slurry is allowed to stand overnight (Note 3) in a refrigerator, then poured into 500 g. of an ice-water mixture. The reaction mixture is extracted with four 150-ml. portions of diethyl ether, and the combined ethereal extracts are washed successively with four 150-ml. portions of 2 N sulfuric acid, 50 ml. of aqueous saturated sodium hydrogen carbonate, and two 50-ml. portions of water. The ethereal solution is dried over anhydrous sodium sulfate, and the solvent removed on a rotary evaporator at 25°, yielding 76–88 g. (78–91%) of crude (E)-4-hexen-1-yl p-toluenesulfonate as a pale yellow oil (Note 4).
A dry, 2-l., three-necked, round-bottomed flask is equipped with a sealed mechanical stirrer, a thermometer, and a pressure-equalizing dropping funnel fitted with a calcium chloride drying tube. Sodium hydride dispersion (Note 5), [19.2 g. of a 50% w/w suspension in mineral oil, 9.6 g. (0.40 mole) of sodium hydride] is placed in the flask, and 100 ml. of anhydrous pentane is added. After the dispersion has been stirred, the sodium hydride is allowed to settle, and the supernatant liquid is removed with a pipet or a siphon. This washing operation is repeated with two additional 100-ml. portions of pentane, and 400 ml. of anhydrous N,N-dimethylformamide (Note 6) is then added to the reaction flask. To the vigorously stirred suspension of sodium hydride in N,N-dimethylformamide is added, dropwise over 30 minutes, 68 g. (0.60 mole) of ethyl cyanoacetate (Note 7). The mixture is stirred until a clear solution is obtained before all of the previously prepared crude (E)-4-hexen-1-yl p-toluenesulfonate, dissolved in 100 ml. of anhydrous N,N-dimethylformamide (Note 6), is added in one portion. The resulting solution is slowly heated to 100°, with continuous stirring, over a period of 3 hours. During this time the reaction becomes dark red, and crystalline sodium p-toluenesulfonate separates. The mixture is allowed to stand overnight at room temperature, then transferred to a 2-l., one-necked, round-bottomed flask, and most of the solvent is removed on a rotary evaporator. The residual semisolid is mixed with 700 ml. of water and extracted with three 250-ml. portions of ether. The combined ethereal extracts are dried over anhydrous sodium sulfate and concentrated on a rotary evaporator. The residual liquid is fractionally distilled under reduced pressure through a 12-cm. Vigreux column. After removal of a low-boiling forerun, b.p. 54–57° (0.2 mm.), containing mainly ethyl cyanoacetate, 34.5–38.4 g. (46–51%) of colorless ethyl (E)-2-cyano-6-octenoate is collected, b.p. 84–86° (0.2 mm.), nD25 1.4458 (Note 8) and (Note 9).
B. Ethyl 1-cyano-2-methylcyclohexanecarboxylate. A 2-l., three-necked, round-bottomed flask is equipped with a heating mantle, a Teflon®-coated magnetic stirring bar, a 1-l. pressure-equalizing dropping funnel fitted with a Teflon® stopcock (Note 10), a stopper, and a reflux condenser fitted with a nitrogen-inlet tube. The apparatus is flushed with nitrogen, and 200 ml. of freshly distilled cyclohexane and 0.30 g. (0.0012 mole) of benzoyl peroxide are added to the flask. While a static nitrogen atmosphere is maintained in the flask (Note 11), a solution of 5.00 g. (0.0270 mole) of ethyl (E)-2-cyano-6-octenoate in 800 ml. of freshly distilled cyclohexane is added from the dropping funnel to the stirred, refluxing benzoyl peroxide solution over a period of 40 hours (Note 10). Three additional 0.30-g. (0.0012-mole) portions of benzoyl peroxide (total: 1.2 g., 0.0050 mole, (Note 11)), are added at 12-hour intervals during the addition of the unsaturated ester. After the addition of the unsaturated ester and the benzoyl peroxide is complete, the reaction mixture is refluxed with stirring for an additional 20 hours (Note 12). The resulting colorless to pale-yellow solution is concentrated on a rotary evaporator to approximately 200 ml., diluted with 250 ml. of ether, and washed with 100 ml. of aqueous saturated iron(II) sulfate to destroy any unchanged benzoyl peroxide. The organic layer is then washed successively with two 200-ml. portions of aqueous saturated sodium hydrogen carbonate and two 200-ml. portions of water and dried over anhydrous calcium chloride. The solvent is removed on a rotary evaporator, and the residual liquid is fractionally distilled under reduced pressure through a 5-cm. Vigreux column. After separation of a 0.16–0.25-g. (3–5%) forerun, b.p. 63–64° (0.2 mm.), nD25 1.4550–1.4560, containing (Note 13) primarily the cyclized product, 3.68–3.82 g. (74–76%) of colorless ethyl 1-cyano-2-methylcyclohexanecarboxylate is collected, b.p. 64–75° (0.2 mm.), nD25 1.4532–1.4539 (Note 14) and (Note 15).
2. Notes
2.
The checkers employed a commercial sample of
p-toluenesulfonyl chloride (obtained from Eastman Organic Chemicals) without further purification.
3.
The checkers found that a longer period of standing before isolation lowered the yield of the sulfonate ester.
4.
IR (CCl
4) cm.
−1: 1375, 1195, 1185, 975 [(
E)CH=CH], 940.
5.
The submitters employed a dispersion of
sodium hydride in mineral oil obtained from Prolabo, Paris. The checkers employed
17 g. of mineral oil dispersion containing 57% sodium hydride obtained from Alfa Inorganics, Inc.6.
The submitters dried commercial
N,N-dimethylformamide over anhydrous
barium oxide for 5 days, then distilled the solvent at atmospheric pressure, b.p.
155°. The checkers allowed commercial
N,N-dimethylformamide to stand over activated Linde 4A Molecular Sieves for several hours, then decanted the solvent and distilled it under reduced pressure, b.p.
43° (6 mm.).
7.
The checkers employed commercial
ethyl cyanoacetate (purchased from Eastman Organic Chemicals) without purification.
8.
The checkers found this fractional distillation to be simplified if the initial crude product was first subjected to a rapid short-path distillation under reduced pressure to remove the bulk of the dialkylated material and other high molecular weight components.
9.
The spectral properties of the product are as follows; IR (CCl
4) cm.
−1: 2250 (C≡N), 1745 (C=O), 970 [(
E) CH=CH];
1H NMR (CCl
4), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 1.0–2.4 (m, 9H, 3C
H2 and allylic C
H3), 1.31 (t,
J = 7, 3H, OCH
2C
H3), 3.44 [t,
J = 6.5, 1H, C
H(CN)], 4.25 (q,
J = 7, 2H, OC
H2CH
3), 5.1–5.8 (m, 2H, C
H=C
H). GC analysis of the checkers' product on a 5-m. column packed with
ethylene glycol isophthalate on Chromosorb P operated at 188° shows 2 products in the ratio of approximately 95:5, and retention times of 33.8 minutes and 37.0 minutes, respectively.
10.
Because of difficulties in adjusting an ordinary glass stopcock to avoid leakage and to maintain a drop rate that would add the unsaturated cyanoester solution over a 40-hour period, the checkers recommend the use of a funnel equipped with a Teflon® stopcock.
11.
The submitters report this reaction to be a radical chain process that requires less than
0.2 mole of benzoyl peroxide per mole of starting material. The checkers can offer additional evidence of the radical chain nature of the reaction from their finding that the cyclization reaction is almost completely inhibited if the refluxing solution is not protected from atmospheric
oxygen.
12.
The reaction solution can be analyzed to establish complete consumption of the starting unsaturated cyanoester by injecting an aliquot of the reaction solution onto a 5-m. GC column packed with
ethylene glycol isophthalate suspended on Chromosorb P operated at 190°. Under these conditions the retention times of the starting unsaturated cyanoester and the cyclized cyanoester are 31.2 minutes and 28.0 minutes, respectively.
13.
GC analysis
(Note 12) of the forerun indicated the presence of
ethyl 1-cyano-2-methylcyclohexanecarboxylate and minor amounts of five or more lower boiling impurities.
14.
The product, which exhibits a single GC peak
(Note 12), is presumably a mixture of stereoisomers. The spectral properties of the product are as follows; IR (CCl
4) cm.
−1: 2250 (C≡N), 1745 (C=O);
1H NMR (CCl
4), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 0.97 (d,
J = 6.2, 3H, C
H3), 1.0–2.5 [m, 9H, C
H(C
H2)
4], 1.32 (t,
J = 7, 3H, OCH
2C
H3), 4.25 (q,
J = 7, 2H, OC
H2CH
3). The methyl doublet at δ 0.97 is accompanied by a second weak doublet (
J = 6.8 Hz.) at δ 1.02 that is presumably attributable to the second stereoisomer of
ethyl 1-cyano-2-methylcyclohexanecarboxylate; mass spectrum
m/e (relative intensity): 195 (M, 5), 141 (27), 136 (40), 126 (34), 123 (62), 122 (80), 108 (100), 98 (85), 95 (62), 94 (44), 82 (27), 81 (41), 70 (51), 67 (56), 55 (50), 53 (32), 42 (27), 41 (58), 39 (31).
15.
The submitters report that this free radical cyclization was also effected by heating a solution of
5.00 g. (0.256 mole) of ethyl (E)-2-cyano-6-octenoate and
1.25 g. (0.00856 mole) of di-tert-butyl peroxide in 500 ml. of freshly distilled cyclohexane at 140° in an autoclave for 30 hours. The solution was concentrated and the residue was distilled to yield
3.4 g. (
68%) of
ethyl 1-cyano-2-methylcyclohexanecarboxylate.
3. Discussion
Ethyl 1-cyano-2-methylcyclohexanecarboxylate has been prepared by catalytically hydrogenating the Diels-Alder adduct of
butadiene and
ethyl 2-cyano-2-butenoate2 and by the procedure described in this preparation.
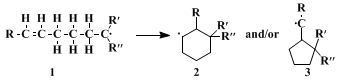
3,4 This procedure illustrates a general method for the preparation of alicyclic compounds by the cyclization of δ-ethylenic carbon radicals
1.
5 Whereas the primary 5-hexen-1-yl radical
1 (R=R'=R"=H) cyclizes to form
methylcyclopentane (
via 3),
6 the 1-cyano-1-carboethoxy disubstituted, radicals
1 (R'=CN, R"=CO
2C
2H
5) lead predominantly, and sometimes exclusively, to six-membered rings
2. Monosubstituted radicals
1 (R"=H) often give mixtures of both isomers
2 and
3.
4,5
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
calcium chloride (10043-52-4)
sulfuric acid (7664-93-9)
ether,
diethyl ether (60-29-7)
hydrogen (1333-74-0)
sodium hydrogen carbonate (144-55-8)
sodium sulfate (7757-82-6)
oxygen (7782-44-7)
barium oxide
nitrogen (7727-37-9)
iron(II) sulfate (13463-43-9)
cyclohexane (110-82-7)
pyridine (110-86-1)
Ethyl cyanoacetate (105-56-6)
Pentane (109-66-0)
benzoyl peroxide (94-36-0)
butadiene (106-99-0)
N,N-dimethylformamide (68-12-2)
sodium hydride (7646-69-7)
methylcyclopentane (96-37-7)
p-Toluenesulfonyl chloride (98-59-9)
Ethyl 1-cyano-2-methylcyclohexanecarboxylate,
Cyclohexanecarboxylic acid, 1-cyano-2-methyl-, ethyl ester (5231-79-8)
ethylene glycol isophthalate
ethyl 2-cyano-2-butenoate
sodium p-toluenesulfonate (657-84-1)
di-tert-butyl peroxide (110-05-4)
(E)-4-Hexen-1-ol (6126-50-7)
(E)-4-hexen-1-yl p-toluenesulfonate
Ethyl (E)-2-cyano-6-octenoate (25143-86-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved