Org. Synth. 1974, 54, 19
DOI: 10.15227/orgsyn.054.0019
γ-HYDROXY-α,β-UNSATURATED ALDEHYDES VIA 1,3-BIS(METHYLTHIO)ALLYLLITHIUM: trans-4-HYDROXY-2-HEXENAL
[2-Hexenal, 4-hydroxy-]
Submitted by Bruce W. Erickson
1
Checked by Susumu Kamata and Wataru Nagata.
1. Procedure
Caution! This preparation requires the use of a good hood.
Methyl iodide, in high concentrations for short periods or in low concentrations for long periods, can cause serious toxic effects in the central nervous system. Accordingly, the American Conference of Governmental Industrial Hygienists
2 has set 5 p.p.m., a level which cannot be detected by smell, as the highest average concentration in air to which workers should be exposed for long periods. The preparation and use of
methyl iodide should always be performed in a well-ventilated fume hood. Since the liquid can be absorbed through the skin, care should be taken to prevent contact.
A. 1,3-Bis(methylthio)-2-propanol. A solution of 44 g. (1.1 moles) of sodium hydroxide in 300 ml. of methanol is placed in a 1-l., four-necked flask equipped with a dry ice reflux condenser, a mechanical stirrer, a thermometer, a gas-inlet, and an ice bath. While the solution is stirred and cooled, 50 g. (56 ml., 1.0 mole) of methanethiol (Note 1) is distilled from a lecture bottle into the solution at such a rate that the temperature is maintained below 20°. The gas-inlet is then replaced with a 60-ml., glass-stoppered, pressure-equalizing dropping funnel, and, while the reaction mixture is stirred and cooled, 44.4 g. (37.6 ml., 0.480 mole) of epichlorohydrin (Note 2) is added dropwise at such a rate that the temperature is maintained below 50° (Note 3).
The reaction mixture is stirred at 25° for 1 hour, diluted with 500 ml. of water, and extracted with two 200-ml. portions of diethyl ether. The combined extract is washed with five 100-ml. portions of water and 100 ml. of saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate (Note 4), filtered, and evaporated at 50° (30 mm.). The residual liquid is distilled under reduced pressure without a column (Note 5), and the fraction boiling at 110–111° (7 mm.) or 101–102° (4 mm.) affords 61.0–63.6 g. (84–87%) of 1,3-bis(methylthio)-2-propanol (Note 6), (Note 7).
B. 1,3-Bis(methylthio)-2-methoxypropane. A dry, 1-l., three-necked flask equipped with a mechanical stirrer, a 60-ml. pressure-equalizing dropping funnel, and a thermometer is charged with 13.8 g. (0.339 mole) of sodium hydride dispersed in mineral oil (Note 8). The mineral oil is removed by washing the dispersion with five 100-ml. portions of hexane (Note 9), which is removed with a pipet after the sodium hydride has been allowed to settle (Note 10).
When most of the hexane has been removed, 500 ml. of dry tetrahydrofuran (Note 11) and (Note 12) is added. While the reaction mixture is stirred, 33.4 g. (0.220 mole) of 1,3-bis(methylthio)-2-propanol is added dropwise over 15 minutes. After the evolution of hydrogen ceases, the stirred mixture is cooled with a water bath. When the mixture reaches 20°, 34.2 g. (15.0 ml., 0.241 mole) of methyl iodide (Note 2) is added dropwise over 5 minutes. The dropping funnel is replaced with a glass stopper, and the reaction mixture is stirred at 20–25° for 24 hours.
The mixture is concentrated to about 200 ml. at 50° (30 mm.), diluted with 200 ml. of ether, and washed with 100 ml. of saturated aqueous sodium chloride, two 100-ml. portions of 0.5 M aqueous ammonium chloride, and 100 ml. of saturated aqueous sodium chloride. Each of the aqueous washes is extracted with the same 100-ml. portion of ether. The combined ethereal solution is dried over anhydrous magnesium sulfate (Note 4), filtered, and evaporated at 25° (10 mm). The residual material is distilled under reduced pressure without a column (Note 5), and the fraction boiling at 96–97° (9 mm.) or 89–90° (7 mm.) affords 31.4–35.7 g. (86–97%) of 1,3-bis(methylthio)-2-methoxypropane (Note 13), (Note 14).
C. 1,3-Bis(methylthio)-1-hexen-4-ol. A dry, 500-ml., three-necked flask containing a Teflon®-coated magnetic stirring bar and equipped with a 100-ml., pressure-equalizing dropping funnel, a thermometer, and a side arm connected to a nitrogen bubbler system is charged with 11.65 g. (0.1153 mole) of diisopropylamine (Note 15) and 175 ml. of dry tetrahydrofuran (Note 11). The flask is flushed with nitrogen, and a slight positive pressure is maintained with a slow stream of nitrogen during the following operation. The solution is magnetically stirred and cooled to about −75° with an acetone-dry ice bath. After 15 minutes, 77.0 ml. (0.112 mole) of 1.45 M solution of n-butyllithium butyllithium in hexane (Note 16) is added dropwise, using the pressure-equalizing dropping funnel. While the resulting solution of lithium diisopropylamide is stirred at −75°, 9.15 g. (0.0551 mole) of 1,3-bis(methylthio)-2-methoxypropane is added through a dropping funnel. The acetone–dry ice bath is replaced with an ice-water bath, and the solution is stirred at 0° for 2.5 hours. The solution slowly becomes deep purple in color as 1,3-bis(methylthio)allyllithium is generated (Note 17).
The solution is stirred and cooled to −75° with an acetone–dry ice bath, and 2.90 g. (0.0500 mole) of propionaldehyde (Note 18) is added through a dropping funnel. The solution is stirred at −75° for 5 minutes. The acetone–dry ice bath is replaced with a water bath, and the solution is stirred at 20° for 30 minutes. The solution is diluted with 250 ml. of ether and washed with two 100-ml. portions of 5 M aqueous ammonium chloride, two 100-ml. portions of water, and 100 ml. of saturated aqueous sodium chloride. Each of the aqueous washes is extracted with the same 250-ml. portion of ether. The combined ethereal solution is dried over anhydrous magnesium sulfate (Note 4), filtered, and evaporated at 40° (25 mm.). The residual liquid is distilled under reduced pressure through a 10 × 0.7 cm., unpacked, vacuum-jacketed column, and the material boiling at 93–98° (2 mm.) furnishes 7.90–7.99 g. (82–83%) of 1,3-bis(methylthio)-1-hexen-4-ol (Note 19), (Note 20).
D. trans-4-Hydroxy-2-hexenal. A 500-ml., one-necked flask containing a Teflon®-coated magnetic stirring bar is charged with 3.85 g. (0.0200 mole) of 1,3-bis(methylthio)-1-hexen-4-ol, 80 ml. of tetrahydrofuran (Note 11), and 6.00 g. (0.0600 mole) of powdered calcium carbonate. The mixture is stirred, and a solution of 16.4 g. (0.0603 mole) of mercury (II) chloride in 140 ml. of tetrahydrofuran and 40 ml. of water is added. The mixture is stirred and heated at 50–55° with a water bath for 15 hours. The reaction mixture is filtered (Note 21) with suction through a pad of diatomaceous earth in a sintered-glass funnel (Note 22). The filter cake is washed with a mixture of 200 ml. of pentane and 200 ml. of dichloromethane. The combined filtrate is washed with three 100-ml. portions of saturated aqueous sodium chloride. Each of the aqueous washes is extracted with a mixture of 100 ml. of pentane and 100 ml. of dichloromethane. The combined organic solution is dried over anhydrous magnesium sulfate (Note 4), filtered, and evaporated at 25° (25 mm.). When almost all the solvent is removed, the residue is slurried with a mixture of 4 ml. of chloroform and 1 ml. of ether as soon as possible. Purification of the product is effected by chromatography on a 3 × 20 cm. column of 50 g. of silicic acid (Note 23) by elution with 4:1 chloroform–ether. Each fraction (50 ml.) is monitored by TLC, and the fractions containing trans-4-hydroxy-2-hexenal uncontaminated with any by-product are collected. The solvent is evaporated at 25° (25 mm. and 1 mm.), yielding 1.37–1.41 g. (60–62%) of product, pure by GC (Note 24) and 1H NMR spectroscopy (Note 25). Distillation without a column yields 1.32 g. (58%) of trans-4-hydroxy-2-hexenal, b.p. 48–51° (0.01–0.03 mm.), 2,4-dinitrophenylhydrazone, m.p. 198–199° (Note 26).
2. Notes
1.
The submitter used
methanethiol (b.p. 8°) obtained from Matheson Gas Products. The weight of the
methanethiol distilled from the lecture bottle into the reaction flask was measured by difference. The pungent odor of this mercaptan is contained by slow distillation of the reagent into the flask and the use of a well-ventilated hood. The checkers used
methanethiol (b.p.
8°,
d40 0.8948) obtained from Toyo Chemical Industries Co. (Japan) and changed the above procedure as follows:
methanethiol distilled from the lecture bottle was first trapped in an ice-cooled graduated flask equipped with a gas-inlet and a dry ice reflux condenser; 56 ml. of the liquid was then redistilled into the reaction mixture.
2.
The submitter used reagent grade material obtained from Eastman Kodak Co., and the checkers used reagent grade material from Kanto Chemical Co., Inc. (Japan).
3.
A large quantity of
sodium chloride precipitates during addition of the
epichlorohydrin.
4.
The checkers used anhydrous
sodium sulfate as a drying agent instead of anhydrous
magnesium sulfate.
5.
The checkers used a
15 × 1 cm., unpacked, vacuum-jacketed column for the distillation.
6.
For GC analysis of the product, the submitter used a
3 ft. × 0.125 in. stainless-steel column of 5% LAC-446 (cross-linked diethylene glycol-adipic ester) on Diatoport S (60–80 mesh), swept with prepurified
nitrogen at 30 ml. per minute. The retention time was 1.75 minutes at 140°. The checkers used a
1 m. × 4 mm. glass column of 5% OV-17 on Chromosorb W (60–80 mesh), swept with prepurified
nitrogen at 90 ml. per minute. The retention time was 2.65 minutes at 150°.
7.
The
1H NMR spectrum (CCl
4) of the product [(CH
3aSCH
2b)
2CH
cOH
d] shows peaks at δ 2.12 (s, 6H,
Ha), 2.63 (d,
Jb,c = 6 Hz., 4H,
Hb), 3.86 (pentet, 1H,
Hc), and 3.78 (s, 1H,
Hd).
8.
Sodium hydride was obtained as a 59% dispersion in mineral oil from Metal Hydrides, Inc.9.
The submitter used a
reagent grade mixture of isomeric hexanes (b.p. 68–70°) obtained from Fisher Scientific Co., and the checkers used the same grade mixture of isomeric hexanes obtained from Wako Pure Chemical Industries Ltd. (Japan).
10.
About 90% of the mineral oil was removed by this procedure. Because some
sodium hydride is lost in the pipet, an excess is initially employed.
11.
The submitter used
tetrahydrofuran obtained from Fisher Chemical Co. and distilled from
lithium aluminium hydride under
nitrogen shortly before use; see
Org. Synth., Coll. Vol. 5, 926 (1973) for warning concerning the purification of
tetrahydrofuran. The checkers used
tetrahydrofuran obtained from Wako Pure Chemical Industries Ltd. (Japan) and distilled from
sodium hydride under
nitrogen shortly before use.
12.
When dry
ether was used in place of dry
tetrahydrofuran, only about 15% of the alcohol was methylated in 36 hours.
13.
Using the columns described in
(Note 6), the retention times were 0.67 minute at 140° and 1.00 minute at 120° (submitter) and 2.30 minutes at 150° and 7.05 minutes at 120° (checkers), respectively.
14.
The
1H NMR spectrum (CCl
4) of the product [(CH
3aSCH
2b)
2CH
cOCH
3d] exhibits absorption at δ 2.13 (s, 6H,
Ha), 2.68 (d,
Jb,c = 5.4 Hz., 4H,
Hb), 3.41 (s, 3H,
Hd), and 3.50 (pentet, 1H,
Hc).
15.
The submitter used
diisopropylamine from Aldrich Chemical Co., and the checkers used
diisopropylamine from Wako Pure Chemical Industries Ltd. (Japan). It was distilled from
calcium hydride before use.
16.
The submitter used a 1.24
M solution of
n-butyllithium in pentane obtained from Foot Mineral Co., and the checkers used a 1.45
M solution of
n-butyllithium in hexane obtained from Wako Pure Chemical Industries Ltd. (Japan). The nominal titer of active alkyl on the bottle agreed well with the value found by titration
3 with 0.80
M (submitter) or 0.50
M (checkers) solution of
2-butanol in
xylene using
1,10-phenanthroline as indicator.
17.
Nearly quantitative generation of
1,3-bis(methylthio)allyllithium was proved, as this solution yielded
1,3-bis(methylthio)propene (
88–89%) and
1,3-bis(methylthio)-1-butene (
89%) by reaction with
methanol and
methyl iodide, respectively. The checkers found that
lithium diisopropylamide can be replaced by
n-butyllithium without any trouble for the generation of
1,3-bis(methylthio)allyllithium, simplifying the procedure considerably at least in this particular case. Subsequent reaction with
propionaldehyde gave
1,3-bis(methylthio)-1-hexen-4-ol in
85% yield, and no appreciable amount of by-product, such as the addition product of
n-butyllithium with
propionaldehyde or with the intermediate
1,3-bis(methylthio)propene, was formed.
18.
The submitter used
propionaldehyde from Aldrich Chemical Co., and the checkers used material obtained from Tokyo Chemical Industry Co. Ltd. (Japan). It was distilled from
calcium hydride shortly before use.
19.
For GC analysis of the products, the submitter used a
3 ft. × 0.125 in. stainless-steel column of 5% LAC-446 (cross-linked diethylene glycol-adipic ester) on Diatoport S (60–80 mesh), heated at 140° and swept with prepurified
nitrogen at 30 ml. per minute. The four isomers were observed as three peaks at retention times of 3.08, 3.69, and 4.07 minutes. The checkers used a
1 m. × 4 mm. glass column of 10% DEGS on Gas Chrome Q (60–80 mesh), heated at 200° and swept with prepurified
nitrogen at 75 ml. per minute. The four isomers were observed at retention times of 3.30, 3.85, 4.10, and 5.20 minutes.
20.
The
1H NMR spectrum (CCl
4) of the product [CH
a(SCH
3b)=CH
cCH
d(SCH
3e)-CH
f(OH)CH
2gCH
3h] exhibits absorption at δ 0.94 (t,
Jg,h = 7 Hz., 3H,
Hh), 1.4 (m, 2H,
Hg), 2.00, 2.03, and 2.05 (s, 3H,
He), 2.22 and 2.26 (s, 3H,
Hb), 2.6–3.8 (m, 2H,
Hd and
Hf), 5.0–5.8 (m, 1H,
Hc), and 5.9–6.3 (m, 1H,
Ha). The olefinic multiplets indicate
4 that two
trans diastereomers and two
cis diastereomers are present in the ratios 25:23:41:11, respectively.
21.
At this stage, the submitter added
2 g. of sodium hydrogen carbonate to buffer the liquid phase near neutrality before filtration. The checkers found that this operation can be omitted without any trouble.
22.
Diatomaceous earth is used to avoid clogging the sintered glass filter with the insoluble material in the reaction mixture.
23.
To remove the remaining
mercury(II) chloride and some by-products the submitter filtered a
carbon tetrachloride solution of the product through a
1-cm. column of Merck silicic acid in an experiment of one-twentieth scale. For the larger-scale preparation the checkers found it necessary to carry out the chromatographic purification using
silicic acid (M. Woelm Eshwege, Grade II) as described.
24.
Using the same columns described in
(Note 19), the product shows a retention time of 1.23 minutes at 140° (submitter) and 1.45 minutes at 200° (checkers).
25.
The
1H NMR spectrum (CCl
4) of the γ-hydroxy-α,β-unsaturated aldehyde [O=CH
aCH
b=CH
cCH
d(OH)CH
2eCH
3f] shows absorption at δ 0.96 (t, 3H,
Hf), 1.2–1.9 (m, 2H,
He), 2.22 (s, 1H, O
H), 4.30 (m, 1H,
Hd), 6.23 (d of d, 1H,
Hb), 6.85 (d of d, 1H,
Hc), and 9.49 (d, 1H,
Ha), with coupling constants
J in Hz.:
Ja,b = 7.6,
Jb,c = 15.5,
Jb,d = 1.0,
Jc,d = 4.3, and
Je,f = 7.0. IR (CCl
4) cm
−1: 3615 medium, 3470 medium and broad (OH), 2805 weak, 2720 weak (CH of aldehyde), 1693 strong (C=O), 1640 weak (C=C).
26.
trans-4-Hydroxy-2-hexenal is unstable and decomposed on standing at room temperature, giving polymeric and dehydrated compounds. The checkers prepared the
2,4-dinitrophenylhydrazone, m.p.
198–199°,
5 for its identification in the usual way.
3. Discussion
1,3-Bis(methylthio)-2-methoxypropane is important as the precursor of
1,3-bis(methylthio)allyllithium,
6 a symmetrical nucleophile synthetically equivalent to the currently unknown (and probably intrinsically unstable) 3-lithio derivative of acrolein (Li-CH=CH-CH=O).
Reaction of
1,3-bis(methylthio)-2-methoxypropane with
2 moles of lithium diisopropylamide6 (or
n-butyllithium) effects (a) the elimination of
methanol, forming
1,3-bis(methylthio)propene, and (b) the lithiation of this
propene, generating
1,3-bis(methylthio)allyllithium in solution. Its conjugate acid,
1,3-bis(methylthio)propene, can be regenerated by protonation with
methanol, and has also been prepared (a) in
31% yield by reaction of
methylthioacetaldehyde with the lithio derivative of diethyl methylthiomethylphosphonate,
6 (b) in a low yield by acid-catalyzed pyrolysis of
1,1-bis(methylthio)-3-methoxypropane,
7 and (c) in low yield by acid-catalyzed coupling of
vinyl chloride with
chloromethyl methyl sulfide.
8A variety of α,β-unsaturated aldehydes are available by alkylation of
1,3-bis(methylthio)allyllithium and hydrolysis of the product. For example,
trans-2-octenal is obtained in 75% yield overall on alkylation with
1-bromopentane and hydrolysis with
mercuric chloride.
6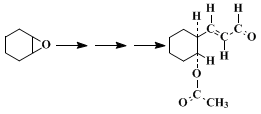
Addition of
1,3-bis(methylthio)allyllithium to aldehydes, ketones, and epoxides followed by mercury(II) ion-promoted hydrolysis furnishes hydroxyalkyl derivatives of acrolein
6 that are otherwise available in lower yield by multistep procedures. For example, addition of
1,3-bis(methylthio)allyllithium to
acetone proceeds in 97% yield, giving a tertiary alcohol that is hydrolyzed with
mercury(II) chloride and
calcium carbonate to
trans-4-hydroxy-4-methyl-2-pentenal in 41% yield.
6 Addition to an epoxide and hydrolysis affords a δ-hydroxy-α,β-unsaturated aldehyde.
9 Similarly, addition of
1,3-bis(methylthio)allyllithium to an epoxide, acetylation of the hydroxyl group, and hydrolysis with
mercury(II) chloride and
calcium carbonate provides a δ-acetoxy-
trans-α,β-unsaturated aldehyde,
6 as indicated in Table I. Cyclic
cis-epoxides give aldehydes in which the acetoxy group is
trans to the 3-oxopropenyl group.
TABLE I
CONVERSION OF EPOXIDES INTO δ-ACETOXY-trans-α,β-UNSATURATED ALDEHYDES
|
|
|
Coupling
|
Acetylation
|
Hydrolysis
|
Overall
|
|
Epoxide
|
Yield, %
|
|
|
Propylene oxide
|
97
|
100
|
81
|
78
|
|
Cyclopentene oxide
|
99
|
92
|
59
|
54
|
|
Cyclohexene oxide
|
96
|
100
|
85
|
82
|
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
hexanes
2,4-dinitrophenylhydrazone
methanol (67-56-1)
ether,
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
hydrogen (1333-74-0)
sodium hydroxide (1310-73-2)
chloroform (67-66-3)
sodium hydrogen carbonate (144-55-8)
Epichlorohydrin (106-89-8)
Propene (115-07-1)
sodium chloride (7647-14-5)
sodium sulfate (7757-82-6)
carbon tetrachloride (56-23-5)
Propionaldehyde (123-38-6)
propylene oxide (75-56-9)
nitrogen (7727-37-9)
calcium carbonate (471-34-1)
acetone (67-64-1)
mercuric chloride,
mercury(II) chloride,
mercury (II) chloride (7487-94-7)
Cyclohexene oxide (286-20-4)
xylene (106-42-3)
Methyl iodide (74-88-4)
Pentane (109-66-0)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
methanethiol (74-93-1)
butyllithium,
n-butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
lithium aluminium hydride (16853-85-3)
sodium hydride (7646-69-7)
hexane (110-54-3)
silicic acid (7699-41-4)
vinyl chloride (9002-86-2)
calcium hydride (7789-78-8)
2-Butanol (78-92-2)
lithium diisopropylamide (4111-54-0)
1,10-phenanthroline (66-71-7)
diisopropylamine (108-18-9)
1,3-BIS(METHYLTHIO)ALLYLLITHIUM
2-Hexenal, 4-hydroxy-
1,3-Bis(methylthio)-2-propanol (31805-83-1)
1,3-Bis(methylthio)-2-methoxypropane (31805-84-2)
1,3-Bis(methylthio)-1-hexen-4-ol (53107-07-6)
1,3-bis(methylthio)propene
1,3-bis(methylthio)-1-butene
methylthioacetaldehyde
1,1-bis(methylthio)-3-methoxypropane
chloromethyl methyl sulfide (2373-51-5)
1-bromopentane (110-53-2)
Cyclopentene oxide (285-67-6)
trans-4-Hydroxy-2-hexenal (17427-21-3)
trans-2-octenal
trans-4-hydroxy-4-methyl-2-pentenal
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved