Org. Synth. 1972, 52, 115
DOI: 10.15227/orgsyn.052.0115
REACTION OF ARYL HALIDES WITH π-ALLYLNICKEL HALIDES: METHALLYLBENZENE
[Benzene, (2-methyl-2-propenyl)-]
Submitted by Martin F. Semmelhack
1 and Paul M. Helquist
2.
Checked by Bradley E. Morris and Richard E. Benson.
1. Procedure
Caution! Nickel carbonyl is a flammable, volatile (b.p. 43°), highly toxic reagent. Safety glasses, gloves, and an apron should be worn when handling this reagent and the first step of this preparation should be conducted in an efficient hood (Note 1).
Benzene has been identified as a carcinogen; OSHA has issued emergency standards on its use. All procedures involving benzene should be carried out in a well-ventilated hood, and glove protection is required.
A 1-l., three-necked flask is equipped with a reflux condenser, a pressure-equalizing dropping funnel, a three-way stopcock, and a large magnetic stirring bar. The system is flushed with argon (Note 2), and 380 ml. of benzene (Note 3) is placed in the flask. From an inverted lecture cylinder 50.8 g. (38.5 ml., 0.298 mole) of nickel carbonyl (Note 4) is introduced into the addition funnel. The nickel carbonyl is added to the benzene while an atmosphere of argon is maintained, and the flask is immersed in an oil bath at 50°. With a syringe, 10.04 g. (0.07437 mole) of methallyl bromide (Note 5) is added over 10 minutes. After a short induction period, evolution of carbon monoxide becomes rapid and a deep red color appears. The exit gas is led from the top of the condenser through a gas bubbler tube to monitor the rate of gas evolution. As the gas evolution becomes vigorous, the bath temperature is raised to 70° and maintained at this temperature for 30 minutes after gas evolution ceases (the total time after addition of methallyl bromide is 1.5 hours). The resulting solution is allowed to cool to 25°, and the benzene and excess nickel carbonyl are removed under reduced pressure (water aspirator), applying an oil bath at 30° as needed to maintain a rapid rate of evaporation (Note 6). The residual, red, solid π-methallylnickel bromide (>85%) is used directly in the next step (Note 7).
A solution of 9.95 g. (0.0634 mole) of bromobenzene (Note 8) in 100 ml. of oxygen-free N,N-dimethylformamide is added, under an argon atmosphere at 25°, to a solution of the crude nickel complex (an 85% yield is assumed) in 65 ml. of oxygen-free N,N-dimethylformamide, over a 15-minute period. After the addition is complete, the reaction mixture is stirred at 25° for 12 hours, then warmed to 60° for one hour. Complete reaction of the nickel complex is indicated by a red to emerald green color change, characteristic of a solution of nickel dibromide in N,N-dimethylformamide. After being cooled to 25°, the solution is poured into a mixture of 250 ml. of water and 250 ml. of petroleum ether (b.p. 30–60°); 2 ml. of 12 M hydrochloric acid is added (Note 10), and the mixture is filtered through Celite filter aid, facilitating separation of the layers. The organic layer is separated, washed with two 100-ml. portions of water, dried over anhydrous magnesium sulfate, and concentrated with a rotary evaporator at water aspirator pressure, affording 8.0–9.6 g. of a clear, colorless liquid. Distillation through a short Vigreux column gives 5.58–6.02 g. (67–72% yield based on bromobenzene) of methallylbenzene as a colorless liquid, b.p. 67–68° (19 mm.), n25D 1.5064 (Note 11).
2. Notes
1.
The treatment for
nickel carbonyl poisoning involves intramuscular injection of BAL (
2,3-dimercapto-1-propanol).
32.
Argon is preferred by the submitters for its high density which allows opening of the reaction vessel without significant displacement of the inert atmosphere by air. A
nitrogen atmosphere was used by the checkers and was equally effective in preventing oxidation of the π-allylnickel complex.
3.
Anhydrous, air-free
benzene was prepared by distillation under
argon, discarding a 20% forerun. The checkers used
benzene from a freshly opened bottle (Fisher Scientific Company).
4.
Nickel carbonyl, available from Matheson Gas Products, was used by the checkers.
5.
Methallyl bromide is prepared from
methallyl chloride (Eastman Organic Chemicals) with a halide exchange reaction. A solution of
148.3 g. (1.639 moles) of methallyl chloride and
213.8 g. (2.460 moles) of lithium bromide in
1 l. of dry acetone is refluxed for 5 hours. The mixture is filtered, and the filtrate is distilled through a
30-cm. Vigreux column, affording
69.2 g. (
31.3%) of
methallyl bromide, b.p.
88–93°,
n25D 1.4672. The purity was 98 ± 2% by GC analysis on a
20% Carbowax column at 65°.
6.
Nickel carbonyl is drawn into the aspirator flow during this operation. In many laboratories the
hood plumbing is connected with the general plumbing line, and vapors of highly toxic
nickel carbonyl may diffuse back to sinks at the laboratory bench. If such an arrangement is suspected, the solvent and excess
nickel carbonyl can be collected by employing a cold
trap (−78° or −196°) between the reaction mixture and the aspirator. Care should be used in the disposal of this mixture.
7.
Pure
π-methallylnickel bromide can be obtained by dissolving the residue in
150 ml. of oxygen-free anhydrous diethyl ether, filtering under
argon, concentrating the filtrate until crystals begin to form, and cooling at −78° for 12 hours. The crystals are isolated by removing the liquid
via suction through a
syringe needle under a positive pressure of
argon, yielding
12.1 g. (
85%) of dark-red crystals. The
1H NMR spectrum can be obtained only by rigorous exclusion of
oxygen from the sample and filtration of the sample as the last stage of sample preparation. The
1H NMR spectrum (C
6D
6) shows three singlets at δ 2.07 (3H), 2.82 (2H), and 2.83 (2H).
8.
Bromobenzene was used as supplied by Aldrich Chemical Company, Inc. Purification by distillation under
argon did not change the yield of
methallylbenzene. The checkers used the product available from Eastman Organic Chemicals (white label).
9.
N,N-Dimethylformamide was distilled from
calcium hydride at 71° (32 mm.) and stored under
argon. The checkers used a freshly opened bottle of the product (white label grade) available from Eastman Organic Chemicals.
10.
The
hydrochloric acid solution is added to speed solution of the nickel salts, which would otherwise lead to emulsions during separation. If no emulsion is encountered after mixing the petroleum ether and water solutions, no
hydrochloric acid need be added. Similarly, the filtration through
Celite filter aid is intended to remove finely divided
nickel metal and other insoluble particles which complicate the washing procedure. If no particles are present, the filtration step should be omitted.
11.
The product consists of
99% methallylbenzene and
1% 2,5-dimethyl-1,5-hexadiene, by
1H NMR analysis. The
1H NMR spectrum of
methallylbenzene (CCl
4) shows peaks at δ 1.63 (broad s, 3H, allylic C
H3), 3.25 (broad s, 2H, allylic C
H2), 4.75 (m, 2H, C=C
H2), and 7.15 (s, 5H, C
6H5). The
1H NMR spectrum of
2,5-dimethyl-1,5-hexadiene (CCl
4) shows peaks at δ 1.70 (broad s, 6H, 2C
H3), 2.12 (broad s, 4H, 2 allylic C
H2), and 4.75 (m, 4H, 2C
H2=C). A small forerun contained
0.30 g. (
3% yield) of
methallylbenzene and a larger quantity of
2,5-dimethyl-1,5-hexadiene. The distillation residue is composed of
0.24–0.34 g. (
3–4% yield) of
methallylbenzene and
0.38–0.52 g. (
8–10% yield) of
biphenyl. The distillation fractions may be analyzed by GC with a
180 cm. by 6.4 mm. column packed with 10% SE-30 on Chromosorb G. The retention times for
methallylbenzene and
2,5-dimethyl-1,5-hexadiene are 4.0 minutes and 2.0 minutes, respectively, at 125°.
3. Discussion
The simple example outlined above, replacement of halogen by a methallyl group, could be carried out in an equally direct way using
phenylmagnesium bromide and methallyl halide. However, the Grignard reaction is complicated by formation of the conjugated, isomeric
β,β-dimethylstyrene,
4 or by a rearrangement to
trans-2-butenylbenzene.
5 In no case has this approach afforded
methallylbenzene in greater than
50% yield. Dehydration of
(2-hydroxy-2-methylpropyl)benzene also produces a mixture of
methallylbenzene (
68%) and
β,β-dimethylstyrene (
32%).
6 Elimination of
benzoic acid from the benzoate ester of (2-hydroxy-2-methylpropyl)benzene gives the same ratio of products, although the combined yield (86%) is lower.
7 The Wittig reaction of
methylenetriphenylphosphorane with
1-phenyl-2-propanone produces
methallylbenzene in only
2% yield.
8The preparation illustrates the procedure for formation of π-allylnickel halides and their reaction with aryl halides.
9 The complexes can be obtained from allylic chlorides, bromides, and iodides
10,11,12 even when the allylic halides bear alkyl, carboalkoxyl, or alkenyl side chains.
11 The coupling step is generally applicable to aryl, alkyl, and vinyl bromides or iodides;
9 organic chlorides are usually unreactive with π-allylnickel halides. Other polar aprotic solvents (
hexamethylphosphoric triamide, dimethyl sulfoxide, N-methylpyrrolidone) have been used.
13 Protic solvents lead to the destruction of the π-allylnickel complex by slow protonation of the allyl ligand.
13 No reaction occurs between aryl, alkyl, or vinyl halides and π-allylnickel bromide in less polar solvents such as
tetrahydrofuran,
1,2-dimethoxyethane,
ether, or hydrocarbons. The π-allylnickel bromide complexes are very reactive with allyl halides, but halogen–metal exchange precedes coupling and a mixture of products is obtained as illustrated in the following example.
14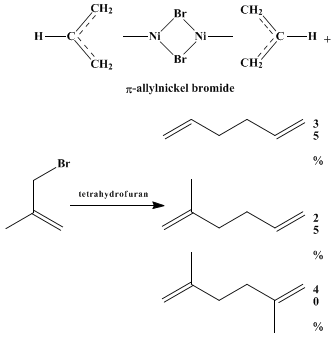
The π-allylnickel complex from
trans-geranyl bromide reacts with alkyl halides, giving a mixture of
cis and
trans products,
9 the double bond that participates in the π-allyl group is isomerized during the sequence of reactions:
Similarly,
trans-4-iodocyclohexanol reacts with
π-methallylnickel bromide, producing a mixture of epimeric 4-methallylcyclohexanols.
9 Note that the hydroxyl group has no significant effect on this reaction.
The advantages of π-allylnickel halides reside in their nonnucleophilic and nonbasic character which allow especially selective reactions with organic halides in the presence of a large number of other functional groups. Carbonyl and hydroxyl groups react much more slowly than organic halides (especially iodides) with π-allylnickel halides, while olefins, nitriles, alkyl chlorides, and aromatic hydrocarbons are inert to these reagents.
9,13
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
petroleum ether
π-methallylnickel bromide
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
ether,
diethyl ether (60-29-7)
carbon monoxide (630-08-0)
oxygen (7782-44-7)
nitrogen (7727-37-9)
Benzoic acid (65-85-0)
nickel (7440-02-0)
acetone (67-64-1)
bromobenzene (108-86-1)
Biphenyl (92-52-4)
Phenylmagnesium bromide (100-58-3)
1-phenyl-2-propanone (103-79-7)
magnesium sulfate (7487-88-9)
Tetrahydrofuran (109-99-9)
nickel carbonyl
N,N-dimethylformamide (68-12-2)
dimethyl sulfoxide (67-68-5)
argon (7440-37-1)
calcium hydride (7789-78-8)
1,2-dimethoxyethane (110-71-4)
nickel dibromide
methallyl chloride (563-47-3)
hexamethylphosphoric triamide (680-31-9)
Methallylbenzene,
Benzene, (2-methyl-2-propenyl)- (3290-53-7)
β,β-dimethylstyrene (768-49-0)
N-methylpyrrolidone (872-50-4)
trans-geranyl bromide (6138-90-5)
methallyl bromide (1458-98-6)
2,3-dimercapto-1-propanol (59-52-9)
lithium bromide (7550-35-8)
2,5-dimethyl-1,5-hexadiene (627-58-7)
(2-hydroxy-2-methylpropyl)benzene (100-86-7)
methylenetriphenylphosphorane (3487-44-3)
trans-2-butenylbenzene (1560-06-1)
trans-4-iodocyclohexanol
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved