Org. Synth. 1983, 61, 65
DOI: 10.15227/orgsyn.061.0065
2-HYDROXYMETHYL-2-CYCLOPENTENONE
Submitted by Amos B. Smith III, Stephen J. Branca, Michael A. Guaciaro, Peter M. Wovkulich, and Abner Korn
1.
Checked by F. Pigott and G. Saucy.
1. Procedure
A.
2-Bromo-2-cyclopentenone. In a
well-ventilated hood, a solution of
18.98 g (231.2 mmol) of 2-cyclopentenone (Note 1) in
150 mL of carbon tetrachloride is added to a
1-L, three-necked, round-bottomed flask fitted with a
mechanical stirrer,
thermometer, and an addition funnel. The solution is chilled to 0°C with an
ice bath and a solution of
40.5 g (253.4 mmol, 13.0 mL) of bromine in 150 mL of carbon tetrachlorideis added dropwise during 1 hr. Then a solution of
35.1 g (346.8 mmol, 48.3 mL) of triethylamine in 150 mL of carbon tetrachloride is added dropwise over 1 hr with vigorous stirring while the reaction is held at 0°C. Stirring is continued for an additional 2 hr at room temperature; the resulting dark suspension is filtered with suction and the filtercake washed with
carbon tetrachloride. The filtrate and washings are combined and washed with two
100-mL portions of 2 N hydrochloric acid, one 100-mL portion of saturated sodium bicarbonate solution, one 100-mL portion of water, and one 100-mL portion of saturated sodium chloride solution. The resultant solution is dried over anhydrous
magnesium sulfate, filtered, and the solvent removed under reduced pressure. Distillation of the resultant oil (69–78°C, 1.0 mm) afforded
23.7 g (147.2 mmol,
64%)
(Note 2) of a white crystalline solid, mp
36–37°C, (lit.
2 mp
39–39.5°C)
(Note 3).
B. 2-Bromo-2-cyclopentenone ethylene ketal. A solution of 22.00 g (136.7 mmol) of freshly distilled 2-bromo-2-cyclopentenone, 21.80 g (351.2 mmol) of ethylene glycol, 1.5 L of benzene (Note 4), and 60 mg of p-toluenesulfonic acid monohydrate is heated at reflux for 64 hr (Note 5), with azeotropic removal of water, in a 3-L, round-bottomed flask, equipped with a Dean-Stark trap, condenser, and Drierite drying tube. The solution is cooled to room temperature, dried with potassium carbonate, and filtered by vacuum through 15 g of Celite. The filtercake is washed with 150 mL of benzene. Removal of the solvent under reduced pressure yields a mobile yellow oil. Distillation (65–67°C, 0.7 mm) affords 22.4 g (109.0 mmol, 80%) (Note 6) of the ketal (Note 7).
C.
2-Hydroxymethyl-2-cyclopentenone. The apparatus, as illustrated in
Figure 1 (Note 8), is flame-dried while dry
nitrogen is passed through.
Paraformaldehyde (13 g, 433 mmol) (Note 9) is then added to the
250-mL flask (the generator) and
19.4 g (94.6 mmol) of freshly distilled 2-bromo-2-cyclopentenone ethylene ketal in
300 mL of dry tetrahydrofuran containing 2 mg of 2,2'-bipyridyl (Note 10) is added to the
500-mL flask (the reaction flask). The reaction flask is chilled to −78°C with an
acetone/dry ice bath and
46.0 mL (105.8 mmol) of butyllithium (2.3 M in hexane)
(Note 11) is added dropwise by syringe through the
rubber septum during 1 hr. The resultant red solution is stirred for 1 hr and then warmed to −30°C using a
methanol–water (2 : 3)/dry
ice bath. An
oil bath previously heated to 160°C is applied to the generator and the monomeric
formaldehyde thus generated is bubbled into the reaction mixture via a steady stream of dry
nitrogen until the red color of the indicator is discharged (approximately 45 min). The reaction is quenched by the addition of
10 mL of saturated ammonium chloride solution and the resultant mixture is poured into
100 mL of a saturated sodium chloride solution. This mixture is extracted four times with
100-mL portions of methylene chloride; the
methylene chloride extracts are combined and dried over
magnesium sulfate. After filtration, evaporation of the solvent under reduced pressure affords
8.1–10.7 g (Note 12) of crude
2-hydroxymethyl-2-cyclopentenone ethylene ketal as a viscous liquid
(Note 13).
Figure 1
Without purification, 8.1 g of the above crude ketal is added to a solution consisting of 1.0 g of oxalic acid, 5 mL of water, and 40 mL of methylene chloride. The resultant mixture is stirred for 5 hr at room temperature. At the end of this period the solution is filtered through 50 g of magnesium sulfate impregnated with 1.0 g of potassium carbonate (Note 14). Evaporation of the solvent from the filtrate affords a solid which, after purification by short-path distillation (70–80°C, 0.1 mm) (Note 15), gives 4.9 g (46%, based on bromoketal) (Note 16) of pure 2-hydroxymethyl-2-cyclopentenone, mp 68–69°C (off-white crystals) (Note 17) and (Note 18).
2. Notes
1.
Cyclopentenone is commercially available from the Aldrich Chemical Company, Inc., or may be prepared according to the procedure of DePuy; see
Org. Synth., Coll. Vol. V 1973, 326.
2.
The checkers obtained somewhat higher yields (i.e.,
66 and 77%).
3.
Pure
2-bromo-2-cyclopentenone obtained by recrystallization from
diethyl ether-hexane2 displayed the following spectroscopic properties: IR (CCl
4) cm
−1: 1720 (s), 1595 (m);
1H NMR (CCl
4, 60 MHz) δ: 2.35–2.60 (m, 2H), 2.60–2.91 (m, 2 H), 7.40 (t, 1 H,
J = 2).
4.
Caution: Benzene is a potential carcinogen!5.
The reaction progress was monitored by TLC analysis (silica gel) using
hexane-
ethyl acetate (4 : 1, v/v) with 3.5% methanolic
phosphomolybdic acid as indicator: bromoketal,
Rf 0.37,
bromoketone,
Rf 0.15.
6.
The bromoketal appears to be somewhat unstable and should be used as soon as possible after preparation. Some decomposition was observed during distillation.
7.
Pure
2-bromo-2-cyclopentenone ethylene ketal displayed the following spectroscopic properties: IR (CCl
4) cm
−1: 2975 (s), 2950 (s), 2880 (s), 1615 (w);
1H NMR (CCl
4, 60 MHz) δ: 1.95–2.55 (m, 4 H), 3.71–4.01 (m, 2 H), 4.01–4.33 (m, 2 H), 6.05 (t, 1 H,
J = 2).
8.
The checkers found that it is important to employ tubing of wide bore (6-mm o.d.) to conduct the gaseous
formaldehyde from the generation flask into the reaction flask to avoid the possibility of the tube becoming plugged.
9.
Prior to use paraformaldehyde was dried overnight in high vacuum (0.1 mm) over
phosphorus pentoxide.
10.
The reagent
2,2'-bipyridyl, available from the Aldrich Chemical Company, Inc., appears red in solutions containing organolithium and organomagnesium reagents
3 and is thereby an excellent indicator. Its use here allows addition of the precise amount of gaseous
formaldehyde.
11.
Butyllithium is available commercially from Alfa Products, Morton Thiokol, Inc.12.
The checkers found this crude product to contain 84.5% of the desired ketal, based on GC analysis.
13.
Although
2-hydroxymethyl-2-cyclopentenone ethylene ketal could be purified by Kugelrohr distillation (88–100°C, 0.10 mm) this was not necessary for successful completion of the subsequent hydrolysis step. Pure
2-hydroxymethyl-2-cyclo-pentenone ethylene ketal possesses the following spectroscopic properties: IR (CCl
4) cm
−1: 3470–3500 (s), 1616 (w);
1H NMR (CCl
4, 60 MHz) δ: 1.68–2.17 (m, 2 H), 2.17–2.58 (m, 2 H), 2.58–2.93 (br s, 1 H), 3.87 (s, 4 H), 3.98–4.16 (m, 2 H), 5.81–6.03 (m, 1 H).
14.
The function of the
potassium carbonate is to neutralize the
oxalic acid as the solution passes through.
15.
The short-path distillation of
2-hydroxymethyl-2-cyclopentenone is carried out without a water condenser. Furthermore, to prevent solidification of the distillate in the condenser, gentle warming of the condenser with a heat gun may be necessary.
16.
The submitters had obtained a 70% yield for this two-step sequence, the crucial step being the reaction with
formaldehyde.
17.
Pure
2-hydroxymethyl-2-cyclopentenone displayed the following spectroscopic properties: IR (CHCl
3) cm
−1: 3400–3450 (s), 1680 (s), 1630 (m);
1H NMR (CDCl
3, 60 MHz) δ: 2.27–2.84 (m, 4 H), 3.00 (br s, 1 H), 4.33 (d, 2 H,
J = 1), 7.60 (m, 1 H).
18.
The overall yield from
cyclopentenone to
2-hydroxymethyl-2-cyclopentenone over a number of runs was found to be in the range of
23–28%. The submitters had obtained
34.5%.
3. Discussion
The procedure reported here provides an efficient method for the construction of a wide variety of α,β-unsaturated ketones directly from the parent enone (i.e.,
1 →
2), which does not require intervention of the thermodynamic dienolate. To our knowledge, a
general solution for this recurring synthetic problem is unavailable, although Corey et al.,
4 Fuchs,
5 and Stork and Panaras
6 have independently developed a reverse polarity (umpolung) strategy for α-arylation of α,β-unsaturated ketones. Central to their approach was the generation of an effective latent equivalent for α-ketovinyl cation
3. Such a strategy, however, is limited in that it depends critically upon the availability of the requisite alkyl or aryl organocuprate or
magnesium reagent.
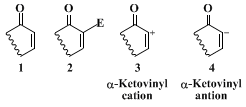
A more versatile, as well as a more direct approach for the conversion of
1 to
2 employs the
ethylene ketal of α-bromo-α,β-enones (e.g.,
5) as a latent equivalent of α-ketovinyl anion
4.
7 Indeed, independent studies by Ficini and Depezay,
8 House and McDaniel,
9 and Manning et al.
10 as well as our own
11 suggested that such a general strategy would be viable. To illustrate this approach, we record here the preparation of the very useful synthon
α-hydroxymethyl-2-cyclopentenone:
The overall efficiency of this sequence demonstrates, we believe, the considerable promise that α-bromoketals of α,β-enones hold as latent α-ketovinyl anion equivalents. In particular, we note the feasibility of introducing the very useful trimethylsilyl, tri-n-butyltin, and phenylselenenyl substituents.
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
potassium carbonate (584-08-7)
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
ethyl acetate (141-78-6)
methanol (67-56-1)
ammonium chloride (12125-02-9)
formaldehyde (50-00-0)
sodium bicarbonate (144-55-8)
magnesium (7439-95-4)
sodium chloride (7647-14-5)
bromine (7726-95-6)
carbon tetrachloride (56-23-5)
Oxalic acid (144-62-7)
nitrogen (7727-37-9)
ethylene glycol (107-21-1)
ethylene (9002-88-4)
methylene chloride (75-09-2)
magnesium sulfate (7487-88-9)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
hexane (110-54-3)
bromoketone (593-95-3)
triethylamine (121-44-8)
tri-n-butyltin (688-73-3)
2-Cyclopentenone,
cyclopentenone (930-30-3)
2,2'-bipyridyl (366-18-7)
trimethylsilyl (16571-41-8)
phenylselenenyl
phosphomolybdic acid (51429-74-4)
2-Hydroxymethyl-2-cyclopentenone (68882-71-3)
2-Bromo-2-cyclopentenone (10481-34-2)
2-Bromo-2-cyclopentenone ethylene ketal (68241-78-1)
2-Hydroxymethyl-2-cyclopentenone ethylene ketal,
2-hydroxymethyl-2-cyclo-pentenone ethylene ketal (80963-19-5)
diethyl ether-hexane
α-hydroxymethyl-2-cyclopentenone
phosphorus pentoxide (1314-56-3)
p-toluenesulfonic acid monohydrate (6192-52-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved