Org. Synth. 1990, 68, 206
DOI: 10.15227/orgsyn.068.0206
IMINIUM ION-BASED DIELS–ALDER REACTIONS: N-BENZYL-2-AZANORBORNENE
[2-Azabicyclo[2.2.1]hept-5-ene, 2-(phenylmethyl)-]
Submitted by Paul A. Grieco and Scott D. Larsen
1.
Checked by V. Ramamurthy and Bruce E. Smart.
1. Procedure
A 100-mL, round-bottomed flask equipped with a Teflon-coated magnetic stirring bar is charged with 24 mL of deionized water and 8.6 g (60.0 mmol) of benzylamine hydrochloride (Note 1). To this homogeneous solution is added 6.3 mL (84 mmol) of 37% aqueous formaldehyde solution (Note 2) followed by 9.9 mL (120 mmol) of freshly prepared cyclopentadiene (Note 3). The flask is stoppered tightly (Note 4) and stirred vigorously at ambient temperature. After 4 hr, the reaction mixture is poured into 50 mL of water and washed with ether–hexane, 1 : 1 (2 × 40 mL). The aqueous phase is made basic by the addition of 4.0 g of solid potassium hydroxide and extracted with ether (3 × 60 mL). The combined ether extracts are dried over anhydrous magnesium sulfate and filtered. The solvent is removed under reduced pressure (15–20 mm) to give 11.2 g (100%) of N-benzyl-2-azanorbornene as a very-pale-yellow oil (Note 5). The crude product is distilled at 80–85°C (0.05 mm) (Note 6) through a short-path apparatus to provide 10.1–10.2 g (91–92%) of pure product (Note 7) as a colorless oil (Note 8).
2. Notes
1.
Benzylamine hydrochloride is commercially available from Aldrich Chemical Company, Inc.2.
Aqueous
formaldehyde solution (37%) is commercially available from Mallinckrodt, Inc.
3.
Cyclopentadiene is prepared by heating commercial
dicyclopentadiene (available from Aldrich Chemical Company, Inc.) at 160°C in a distillation apparatus,.
Cyclopentadiene distills smoothly at 39–45°C.
24.
The heterogeneous reaction mixture is stoppered tightly to avoid loss of
cyclopentadiene.
5.
This crude material is essentially pure product contaminated by trace amounts of
ether.
N-Benzyl-2-azanorbornene has the following spectrum:
1H NMR (300 MHz, CDCl
3) δ: 1.42 (dm, 1 H,
J = 8), 1.52 (dd, 1 H,
J = 2, 8.5), 1.64 (dm, 1 H,
J = 8), 2.94 (bs, 1 H), 3.18 (dd, 2 H,
J = 3, 8.5), 3.34, 3.58 (AB, 2 H,
J = 13), 3.83 (m, 1 H), 6.09 (dd, 1 H,
J = 2, 6), 6.38 (ddd, 1 H,
J = 2, 3, 6), 7.2–7.4 (m, 5 H).
6.
Attempted distillation at 15–20 mm resulted in extensive decomposition.
7.
The submitters obtained
10.8 g (
97%) of analytically pure product, bp
80–85°C (0.05 mm). Anal. calcd. for C
13H
15N: C, 84.28; H, 8.16; N, 7.56. Found: C, 84.68; H, 8.36; N, 7.59.
8.
On prolonged standing in air at room temperature discoloration of the product accompanied by slow evolution of
cyclopentadiene takes place.
3. Discussion
Simple unactivated iminium salts generated in situ from
formaldehyde and primary alkyl amines undergo a facile aza Diels–Alder reaction with
cyclopentadiene at ambient temperature
3 to afford novel
N-alkylated 2-azanorbornenes. The procedure described above is general and can be applied to a number of primary alkyl amines. Yields of
N-alkyl substituted 2-azanorbornenes are good to excellent. Use of
ammonium chloride and
formaldehyde in the above reaction produces
2-azanorbornene in modest (
40–50%) yield.
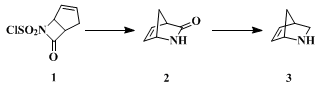
2-Azanorbornene (3) has been previously prepared
4 by reaction of
cyclopentadiene with
chlorosulfonyl isocyanate, which provides a single
N-chlorosulfonyl β-lactam
(1). Exposure of
1 to an aqueous solution of
sodium sulfite gives rise (
25–30%) to
2-azanorbornen-3-one (2) which upon reduction with
lithium aluminum hydride affords (ca.
80%)
2-azanorbornene (3).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
ether (60-29-7)
ammonium chloride (12125-02-9)
sodium sulfite (7757-83-7)
formaldehyde (50-00-0)
potassium hydroxide (1310-58-3)
magnesium sulfate (7487-88-9)
lithium aluminum hydride (16853-85-3)
hexane (110-54-3)
CYCLOPENTADIENE (542-92-7)
dicyclopentadiene (77-73-6)
CHLOROSULFONYL ISOCYANATE (1189-71-5)
2-Azabicyclo[2.2.1]hept-5-ene, 2-(phenylmethyl)-,
N-Benzyl-2-azanorbornene (112375-05-0)
benzylamine hydrochloride (3287-99-8)
2-azanorbornene
2-azanorbornen-3-one
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved