Org. Synth. 1990, 69, 220
DOI: 10.15227/orgsyn.069.0220
REARRANGEMENT OF 4-ARYL-4-HYDROXY-2,3-DIALKOXYCYCLOBUTENONES TO ANNULATED HYDROQUINONES AND QUINONES: 5,6-DIETHOXYBENZOFURAN-4,7-DIONE
Submitted by S. T. Perri, P. Rice, and H. W. Moore
1.
Checked by Ho-Jung Kang and Leo A. Paquette.
1. Procedure
A 500-mL, round-bottomed flask is flame-dried and flushed with nitrogen. The flask is equipped with a magnetic stirring bar and a rubber septum and charged with 4.14 g (60.9 mmol) of furan (Note 1) and 300 mL of dry tetrahydrofuran (Note 2). The solution is stirred and cooled in an ethylene glycol–dry ice bath (−15°C) and 24.17 mL (55.6 mmol) of 2.3 M butyllithium is added slowly by means of a syringe pump (rate = 1.5 mL/min). After complete addition, the solution is stirred for an additional 30 min. The ethylene glycol–dry ice bath is replaced with an ice bath and the solution stirred for 1.5 hr at 0°C. The flask is then cooled to −78°C in a dry ice–acetone bath.
A 1000-mL, round-bottomed flask is flame-dried and flushed with nitrogen. The flask is equipped with a magnetic stirring bar and a rubber septum and charged with 9.00 g (52.9 mmol) of diethyl squarate (Note 3) and 450 mL of dry tetrahydrofuran (Note 2). The solution is stirred and cooled in a dry ice–acetone bath at −78°C. The solution of 2-lithiofuran is transferred dropwise via cannula to the flask containing the diethyl squarate, which is stirred rapidly. After complete addition (45 min), the solution is stirred for 20 min and quenched by pouring the cold solution into a separatory funnel containing 150 mL of aqueous 10% ammonium chloride and 100 mL of diethyl ether. The separatory funnel is shaken vigorously until phase separation is achieved and all of the ice has melted. The aqueous phase is separated from the organic phase and the aqueous layer is extracted twice with 40-mL portions of diethyl ether. The combined organic layer is washed with 125 mL of brine solution and dried over solid anhydrous potassium carbonate (Note 4) for 5 min with gentle swirling.
The solution is decanted from the potassium carbonate (Note 5) and concentrated on a rotary evaporator (bath temperature = 23–40°C) to approximately 150 mL in a 1000-mL round-bottomed flask. Then 400 mL of dry p-xylene (Note 6) is added and the flask is placed on the rotary evaporator at a bath temperature of 40°C to remove the remaining tetrahydrofuran and diethyl ether. The flask containing the remaining p-xylene solution of the cyclobutenone is fitted with a reflux condenser and heated at reflux for 3 hr under nitrogen. The flask is cooled to ambient temperature and the solvent is removed on a rotary evaporator fitted with a bump trap at a bath temperature of 70°C to give a red oil.
The oil is dissolved in 500 mL of diethyl ether and washed with three 200-mL portions of an ethanolic ferric chloride solution (Note 7). The aqueous layers are separated from the organic phase and extracted with four 100-mL portions of diethyl ether. The combined organic layer is washed with saturated sodium bicarbonate solution (Note 8) until the aqueous wash is no longer acidic. The resulting neutralized aqueous extracts are combined and extracted with three 30-mL portions of diethyl ether. The organic extracts are combined, washed with 150 mL of brine solution, and dried over magnesium sulfate. The solution is filtered and concentrated on a rotary evaporator to give the quinone as a red solid. The solid is recrystallized from methanol to yield 10.4–10.5 g of the quinone as orange needles in two or three crops (83–84% based on diethyl squarate), mp 54–55°C (Note 9).
2. Notes
1.
Furan was purchased from Aldrich Chemical Company, Inc., and used as such.
2.
Tetrahydrofuran was distilled under
argon from
benzophenone ketyl.
3.
3,4-Diethoxy-3-cyclobutene-1,2-dione is commercially available from Aldrich Chemical Company, Inc. Caution: This substance was found to cause severe skin rashes, and extreme care should be exercised in handling it. The dimethoxy analog appears to be safer.
Diethyl squarate has the following spectral properties: IR (neat) cm
−1: 1830, 1741, 1609;
1H NMR (CDCl
3, 300 MHz) δ: 1.47 (t, 6 H,
J = 2.9), 4.47 (q, 4 H,
J = 7.1).
4.
Prolonged drying of the cyclobutenone over
potassium carbonate resulted in product decomposition. Anhydrous
magnesium sulfate was found to hydrolyze the product during the thermolysis step.
5.
The cyclobutenone was pure enough to use in the next step without purification. This compound is stable for a few hours in solution while kept cold (< 5°C) and anhydrous.
6.
Certified p-xylene was purchased from Fisher Scientific Company and used as such.
7.
Ferric chloride was purchased from Mallinckrodt, Inc. A saturated solution of
80 g of ferric chloride in 500 mL of water was diluted with
500 mL of ethanol, filtered, and used as such. The oxidation could be followed by TLC using Engel stain.
8.
Quinones are generally sensitive to bases, and some decomposition may occur if the product is exposed for a prolonged period of time to
sodium bicarbonate. Therefore the neutralization wash was carried out quickly.
9.
The spectral properties of the product are as follows: IR (CHCl
3) cm
−1: 2980, 1667, 1480, 1370, 1290, 1250, 1179;
1H NMR (CDCl
3, 500 MHz) δ: 1.42 (2 overlapping t, 6 H, two CH
3), 4.30 (2 overlapping q, 4 H, two CH
2), 6.81 (d, 1 H,
J = 1.9), 7.67 (d, 1 H,
J = 1.8);
13C NMR (CDCl
3) δ: 15.7, 15.8, 70.2, 70.3, 108.3, 126.7, 145.7, 146.6, 148.5, 150.1, 172.7, 178.8; EI–MS (70 eV):
m/z = 236 (19%), 208 (21), 193 (8), 180 (24), 163 (7), 152 (100), 123 (9).
3. Discussion
This procedure describes a synthetic route to annulated hydroquinones and quinones. The example represents a general, convergent, regiospecific and usually high-yielding method. This is further elaborated by the generalized scheme given below. Specifically, dialkoxycyclobutenediones (e.g.,
dimethyl squarate,
1) are easily converted to unsymmetric cyclobutenediones
2 on treatment with an organolithium reagent (R = alkyl, aryl, alkenyl, alkynyl, −78°C, THF) followed by treatment with
trifluoroacetic anhydride (−78°C, TFAA) and an aqueous workup.
2,3,4 Treatment of
2 with an aryllithium reagent results in the regiospecific formation of the cyclobutenones
3 via 1,2-addition to the more nucleophilic carbonyl group. These adducts then undergo facile rearrangement to the corresponding annulated hydroquinones in refluxing
p-xylene. The product is usually isolated as the quinone
4 after an oxidative workup.
5,6,7 It is noted that alkenyl lithium reagents as well as alkynyl analogs also add regiospecifically to the cyclobutenediones
2 to give the corresponding cyclobutenones. The alkenyl adducts, like the aryl analogs, also rearrange to the corresponding hydroquinones. The alkynyl adducts rearrange directly to the corresponding quinones via a unique pathway involving migration of the alcoholic proton of the
4-hydroxycyclobutenone to the quinone ring nucleus of the product.
8,9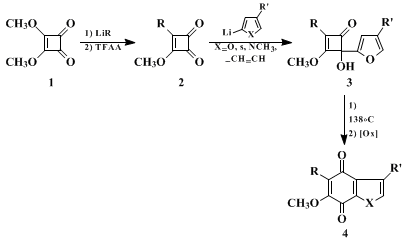
Compounds 5–10 are specific examples of annulated products that have been prepared by the method outlined here.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
benzophenone ketyl
brine
ethanolic ferric chloride
ethanol (64-17-5)
potassium carbonate (584-08-7)
methanol (67-56-1)
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
sodium bicarbonate (144-55-8)
nitrogen (7727-37-9)
ferric chloride (7705-08-0)
p-xylene (106-42-3)
Furan (110-00-9)
magnesium sulfate (7487-88-9)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
argon (7440-37-1)
trifluoroacetic anhydride (407-25-0)
5,6-Diethoxybenzofuran-4,7-dione (138225-13-5)
diethyl squarate,
3,4-Diethoxy-3-cyclobutene-1,2-dione
2-Lithiofuran
4-hydroxycyclobutenone
DIMETHYL SQUARATE (5222-73-1)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved