Org. Synth. 1990, 68, 130
DOI: 10.15227/orgsyn.068.0130
PALLADIUM-CATALYZED REACTION OF 1-ALKENYLBORONATES WITH VINYLIC HALIDES: (1Z,3E)-1-PHENYL-1,3-OCTADIENE
[Benzene, 1,3-octadienyl-, (Z,E)-]
Submitted by Norio Miyaura and Akira Suzuki
1.
Checked by Albert L. Casalnuovo, Thomas S. Kline, Jr., and Bruce E. Smart.
1. Procedure
A.
(E)-1-Hexenyl-1,3,2-benzodioxaborole. A
25-mL, three-necked, round-bottomed flask is equipped with a
magnetic stirring bar, a
thermometer, a
rubber septum, a
10-mL addition funnel, and a
reflux condenser. The apparatus is connected through the condenser to a
nitrogen source and an oil bubbler
(Note 1). The flask is charged with
4.9 g (60 mmol) of 1-hexyne (Note 2) through the addition funnel. While the solution is stirred slowly,
6.7 mL (60 mmol) of catecholborane (Note 3) is injected by syringe through the septum cap. The exothermic reaction is maintained at 60–70°C by intermittent cooling in an
ice–water bath. The reaction mixture is allowed to cool to room temperature and is stirred for 15 min. The rubber septum is replaced by a
glass stopper, and the mixture is heated to 60°C and stirred for an additional 2 hr. The flask is cooled to room temperature, the condenser is replaced by a short-path distillation head, and the mixture is distilled at reduced pressure to give
9.5–10.5 g (
78–87%) of clear, colorless product, bp
75–76°C (0.10 mm) [lit.
2 bp
82°C (0.25 mm)]
(Note 4).
B. (1Z,3E)-1-Phenyl-1,3-octadiene. A 500-mL, three-necked, round-bottomed flask equipped with a magnetic stirring bar, a reflux condenser to which a nitrogen inlet tube and oil bubbler are attached, a glass stopper, and an addition funnel is flushed with nitrogen and charged with 9.5 g (47 mmol) of (E)-1-hexenyl-1,3,2-benzodioxaborole and 200 mL of benzene (Note 5). The solution is stirred and 8.4 g (46 mmol) of (Z)-β-bromostyrene (Note 6), 50 mL of 2 M sodium ethoxide in ethanol (Note 7), and finally 0.28 g (0.4 mmol) of dichlorobis(triphenylphosphine)palladium(II) (Note 8) are added. The mixture is refluxed for 3 hr. The light-brown solution containing a white precipitate of sodium bromide is cooled to room temperature, treated with 60 mL of 3 M sodium hydroxide and 6 mL of 30% hydrogen peroxide, and stirred at room temperature for 1 hr (Note 9). The organic layer is separated, washed 4 times with 50 mL of 3 M sodium hydroxide (Note 10), and dried over anhydrous magnesium sulfate. The drying agent is removed by filtration and the filtrate is concentrated on a rotary evaporator. The residual oil is distilled under reduced pressure (Note 11) to give 7.0 g (82%) of (1Z,3E)-1-phenyl-1,3-octadiene as a clear, colorless liquid, bp 80°C (0.15 mm) (Note 12).
2. Notes
1.
All glassware was predried in an
oven at 130°C for 3 hr, assembled while hot, and allowed to cool under a stream of
nitrogen.
3.
Catecholborane (1,3,2-benzodioxaborole) with a purity of 95% was purchased from Aldrich Chemical Company, Inc. and purified by distillation under
nitrogen, bp
58°C (52 mm). For the distillation and handling of air and moisture-sensitive compounds, see
3,4,5.
Catecholborane is a liquid at room temperature, and the neat material is 9.0
M in
catecholborane.
3 The preparation of
catecholborane from
borane and
catechol has been reported.
34.
The submitters report bp
86–87°C (0.3 mm).
(E)-1-Hexenyl-1,3,2-benzodioxaborole is quite air-stable, but it can slowly hydrolyze to boronic acid and turn brown on repeated use in air. The submitters recommend storing it at
refrigerator temperature in a bottle purged with
nitrogen and capped with a rubber septum. Alternatively, the crude
1-hexenyl-1,3,2-benzodioxaborole can be used the next coupling reaction without purification. In this case, the unreacted
1-hexyne should be removed under reduced pressure (0.1 mm for 30 min), because it also reacts with
(Z)-β-bromostyrene to afford
(1Z)-1-phenyl-1-octen-3-yne. In this manner the expected diene was obtained in a yield of
86%.
5.
Benzene was obtained from Fisher Scientific Company and redistilled before use.
7.
The
sodium ethoxide solution was prepared by dissolving
2.3 g of sodium in
50 mL of anhydrous ethanol and was used immediately.
8.
The
palladium catalyst is prepared as follows. A
100-mL, one-necked, round-bottomed flask equipped with a magnetic stirring bar and a reflux condenser connected to a
nitrogen inlet is flushed with
nitrogen and charged with
1.00 g (5.64 mmol) of palladium chloride (Johnson Matthey, Inc.),
3.25 g (12.4 mmol) of triphenylphosphine (Aldrich Chemical Company, Inc.), and
30 mL of benzonitrile (Aldrich Chemical Company, Inc.). The mixture is stirred, gradually heated to 180°C, and held at that temperature for 20 min. The clear, red solution that results is allowed to cool slowly to room temperature and stand overnight. The bright-yellow crystals that precipitate are collected by filtration, washed with three
10-mL portions of ether, and dried at reduced pressure to give
3.90 g (5.55 mmol) of dichlorobis(triphenylphosphine)palladium(II).
9.
This operation removes most of the palladium-containing compounds. Any unreacted
1-hexenylboronate is oxidized to
hexenal.
10.
Catechol must be washed out completely because it is difficult to remove by distillation. A solution of
catechol in aqueous
sodium hydroxide turns dark brown on treatment with
hydrogen peroxide or on standing in air.
11.
The residual oil can be purified before distillation by filtering it through a short
(20-cm) silica gel column (70–230 mesh) using hexanes as an eluant. This effectively removes traces of
catechol and palladium-containing compounds.
12.
Gas chromatographic analysis of the product (Hewlett-Packard fused silica, crosslinked,
methylsilicone capillary column, 25 m × 20 mm, column temperature 100–270°C, injection temperature 250°C) shows that the product is over
99% chemically and isomerically pure.
(Z,E)-1-Phenyl-1,3-octadiene shows the following spectral properties: IR (neat) cm
−1: 1640, 1595, 1490, 985;
1H NMR (CDCl
3) δ: 0.89 (t, 3 H,
J = 7.1), 1.25–1.45 (m, 4 H), 2.05–2.20 (m, 2 H), 5.87 (d of t, 1 H,
J = 7.1, 15, PhC=C-C=CH), 6.21 (d of d, 1 H,
J = 11.2, 11.6, PhC=CH), 6.30 (d, 1 H,
J = 11.6, PhCH=C), 6.60 (d of d, 1 H,
J = 11.2, 15, PhC=C-CH=C), and 7.15–7.40 (m, 5 H, aromatic).
3. Discussion
The procedure described here is an example of a general method for preparing conjugated alkadienes by the palladium-catalyzed reaction of 1-alkenylboranes or boronates with vinylic halides. Hydroboration of 1-alkynes with
catecholborane is a standard method for obtaining
(E)-1-alkenylboronates (
1).
2,3 Several different types of alkenylboranes and boronates (
2–4) are now available as reagents for the cross-coupling reaction with vinyl halides.
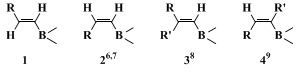
These alkenylboron derivatives react not only with 1-alkenyl halides but also with a variety of other organic halides, including 1-bromo-1-alkynes,
6 aryl halides,
7,8,9 and allylic or benzylic halides,
10 in the presence of a
palladium catalyst and base. Both Pd(PPh
3)
4 and PdCl
2(PPh
3)
2 are excellent catalysts for most of the reactions. A base is generally required for successful coupling.
Sodium ethoxide (2 equiv) in
ethanol–
benzene, which is used in the procedure described here, gives high yields with most 1-bromo-1-alkenes. For 1-iodo-1-alkenes, aqueous
sodium hydroxide in
tetrahydrofuran11 or aqueous 4
M potassium hydroxide (3 equiv) in
benzene8 can give better results. Alkoxides and hydroxides normally accelerate the reaction, but the choice of base depends on its compatibility with the particular organic halide. For the coupling reaction with 3-halo-2-cyclohexen-1-one
12 a relatively weak base, such as
sodium acetate in
methanol, works well. The reaction with 1-bromo-2-phenylthio-1-alkenes
13 is successfully carried out using aqueous
potassium hydroxide. For the reaction of
(E)-2-ethoxyvinylborane with aryl halides,
14 a suspension of
sodium hydroxide in
tetrahydrofuran gives better results than homogeneous base. The versatility of these methods has been reviewed.
15,16In addition to alkenylboron compounds, alkenylalane,
17 18 19 alkenylzirconium,
20 21 alkenyltin,
22 alkenylcopper,
23 24 and alkenylmagnesium
25 reagents are reported to undergo a related alkenyl–alkenyl coupling reaction to give 1,3-alkadienes.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(E)-1-alkenylboronates
ethanol (64-17-5)
Benzene (71-43-2)
methanol (67-56-1)
ether (60-29-7)
sodium acetate (127-09-3)
benzonitrile (100-47-0)
sodium hydroxide (1310-73-2)
sodium bromide (7647-15-6)
nitrogen (7727-37-9)
potassium hydroxide (1310-58-3)
sodium (13966-32-0)
sodium ethoxide (141-52-6)
palladium (7440-05-3)
Catechol (120-80-9)
hydrogen peroxide (7722-84-1)
palladium chloride (7647-10-1)
magnesium sulfate (7487-88-9)
borane (7440-42-8)
Tetrahydrofuran (109-99-9)
1-Hexyne (693-02-7)
triphenylphosphine (603-35-0)
CATECHOLBORANE,
1,3,2-benzodioxaborole (274-07-7)
(Z)-β-Bromostyrene (103-64-0)
dichlorobis(triphenylphosphine)palladium(II) (13965-03-2)
1-hexenyl-1,3,2-benzodioxaborole
1-hexenylboronate
hexenal
(1Z,3E)-1-Phenyl-1,3-octadiene,
(Z,E)-1-Phenyl-1,3-octadiene (39491-66-2)
Benzene, 1,3-octadienyl-, (Z,E)- (39491-66-2)
(E)-1-Hexenyl-1,3,2-benzodioxaborole (37490-22-5)
(1Z)-1-phenyl-1-octen-3-yne
(E)-2-ethoxyvinylborane
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved