Org. Synth. 1987, 65, 150
DOI: 10.15227/orgsyn.065.0150
SYNTHESIS OF MACROCYCLIC SULFIDES USING CESIUM THIOLATES: 1,4,8,11-TETRATHIACYCLOTETRADECANE
Submitted by J. Buter and Richard M. Kellogg
1.
Checked by Joseph M. Salvino and Bruce E. Smart.
1. Procedure
A.
3.7-Dithianonane-1,9-diol.2 A
500-mL, three-necked, round-bottomed flask is fitted with a
mechanical stirrer, a
reflux condenser attached to a
nitrogen inlet, and a
pressure-equalizing dropping funnel. The flask is flushed with
nitrogen and charged with
250 mL of absolute ethanol. The
ethanol is stirred and
5.75 g (0.25 mol) of sodium metal is cautiously added. After the
sodium dissolves, the solution is warmed to 45–50°C and
13.5 g (0.125 mol) of 1,3-propanedithiol (Note 1) is added dropwise over a period of 15 min. To the resulting solution is added dropwise
20.1 g (0.25 mol) of 2-chloroethanol (Note 1) and the mixture is refluxed for 3–4 hr. The mixture is then allowed to cool to room temperature and is filtered. The filtrate is concentrated on a
rotary evaporator to a viscous liquid, which is distilled to give
17.3–20.0 g (
71–82%) of
3,7-dithianonane-1,9-diol, bp
200°C (1.5 mm) (Note 2).
B.
3,7-Dithianonane-1,9-dithiol.2 In a
1-L, round-bottomed flask equipped with a reflux condenser and a
magnetic stirring bar are placed
35.0 g (0.178 mol) of 3,7-dithianonane-1,9-diol,
30.0 g (0.394 mol) of thiourea (Note 3), and
94 mL of concentrated hydrochloric acid. The mixture is stirred and refluxed for 12 hr. The resulting solution is cooled in an
ice bath and
67 g (1.2 mol) of potassium hydroxide dissolved in 400 mL of water is added cautiously. The mixture is then refluxed for 3 hr. The resulting two-phase system is cooled to room temperature and the upper aqueous phase is decanted from the oily organic layer. The aqueous phase is acidified with dilute
hydrochloric acid and extracted with
300 mL of ether. The ethereal extract is combined with the organic layer from the reaction mixture, and this solution is dried over anhydrous
magnesium sulfate. The drying agent is removed by filtration and the filtrate is concentrated on a rotary evaporator. The residual liquid is distilled to give
21.4 g (
53%) of
3,7-dithianonane-1,9-dithiol, bp
159–162°C (1.2 mm) (Note 4).
C.
1,4,8,11-Tetrathiacyclotetradecane. A dry,
3-L, three-necked, round-bottomed flask is equipped with a
double-necked adapter for a thermometer and the
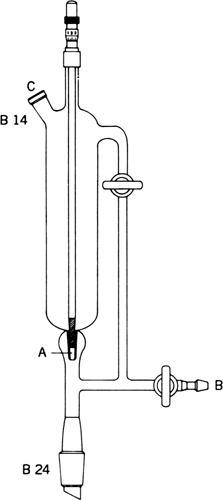
250-mL addition funnel shown in
Figure 1 (Note 5), a reflux condenser, and a
mechanical stirrer with a 7-cm blade of Teflon. The entire system is kept under positive
nitrogen pressure. The flask is charged with
2.2 L of N,N-dimethylformamide and
13.04 g (40 mmol) of cesium carbonate (Note 6). The mixture is stirred and heated to 55–60°C.
Figure 1. Addition funnel.
A solution of
9.12 g (40 mmol) of 3,7-dithianonane-1,9-dithiol and
8.08 g (40 mmol) of 1,3-dibromopropane (Note 6) in
300 mL of N,N-dimethylformamide is prepared. Half of this solution is placed in the addition funnel and added to the well-stirred suspension of
cesium carbonate in
N,N-dimethylformamide over a period of 6–9 hr. The reaction mixture is then charged with another
13.04 g (40 mmol) of cesium carbonate and the second half of the solution of
3,7-dithianonane-1,9-dithiol and
1,3-dibromopropane is added over a period of 6–9 hr
(Note 7). After the addition is complete, the reaction mixture is allowed to cool to room temperature. The
N,N-dimethylformamide is distilled off as completely as possible under reduced pressure
(Note 8). The residue is taken up in
300 mL of dichloromethane and washed once with
200 mL of a saturated solution of sodium chloride. The organic layer is dried over anhydrous
magnesium sulfate. The drying agent is removed by filtration and the filtrate is concentrated on a rotary evaporator to a light-yellow crystalline mass. This is taken up in
200 mL of boiling 95% ethanol and the hot liquid is decanted. The remaining sediment is boiled with
125 mL of 95% ethanol and again decanted. The two
ethanol solutions are combined and stored at 10°C overnight. The white crystalline product that separates is isolated by filtration and dried. The final product obtained is
6.20–6.60 g (
58–62%) of
1,4,8,11-tetrathiacyclotetradecane, mp
118–119°C (lit.
2 mp
119–120°C)
(Note 9).
2. Notes
1.
1,3-Propanedithiol and 2-chloroethanol were obtained from the Aldrich Chemical Company, Inc.2.
The submitters report obtaining
19.6–24.5 g (
80–100%) of product, bp
179–181°C (0.5 mm). The product obtained by the checkers is pure by NMR and shows the following spectrum:
1H NMR (CDCl
3) δ: 1.86 (quintet, 2 H,
J = 7, CCH
2), 2.17 (br s, 2 H, OH), 2.65 (t, 4 H,
J = 7, SCH
2), 2.73 (t, 4 H,
J = 6, SCH
2), 3.73 (t, 4 H,
J = 6, OCH
2).
3.
The checkers purchased
thiourea from the Aldrich Chemical Company, Inc.4.
The submitters report bp
159–161°C (0.5 mm) for the product and yields of
50–70% for reactions run with
0.1 mol of 3,7-dithianonane-1,9-diol and
0.2 mol of thiourea. The product obtained by the checkers is pure by NMR and shows the following spectrum:
1H NMR (CDCl
3) δ: 1.63–2.07 (m, 4 H, CCH
2, SH), 2.50–2.83 (m, 12 H, SCH
2).
5.
The device illustrated in
Figure 1 allows ready adjustment of addition rates without significant clogging. Component A is a
conical ground-glass receiver for the ground-glass tapered end of a 7-mm-diameter glass rod. The rod is turned in the tapered receiver to attain the desired rate of addition. A ratchet device attached to the top of the addition funnel holds the rod in place and measures its rotation. Outlet B is connected to a
mercury bubbler and
nitrogen is introduced via C.
6.
The checkers obtained
N,N-dimethylformamide, cesium carbonate, and 1,3-dibromopropane from the Aldrich Chemical Company, Inc. The
N,N-dimethylformamide was distilled and stored over molecular sieves (Linde 4A) prior to use.
7.
The addition rate is sufficiently slow that virtually no starting material remains. The procedure avoids the need for excessively large volumes of solvent that are normally required in high dilution reactions.
8.
A
Büchi rotary evaporator attached to a
vacuum pump is used. A pressure of 1–2 mm is maintained and the flask is heated in a
water bath to a maximum temperature of 60°C.
9.
The submitters report obtaining
6.97–8.00 g (
65–70%) of product, mp
118–119.5°C. The product shows the following spectral properties: IR (KBr) cm
−1: 2930, 1430, 1338, 1270, 1205, 1138, 692;
1H NMR (CDCl
3) δ: 1.90 (quintet, 4 H,
J = 7, CCH
2), 2.65 (t, 8 H,
J = 7, SCH
2CH
2), 2.77 (s, 8 H, SCH
2).
3. Discussion
The cyclic sulfide
1,4,8,11-tetrathiacyclotetradecane has been prepared in
7.5% yield by reaction of the
bis-Na+ salt of 3,7-dithianonane-1,9-dithiol in boiling ethanol with
1,3-dibromopropane.
2 A marked improvement in yield by the use of cesium salts in
N,N-dimethylformamide has been documented for other macrocyclic sulfides,
3 as well as macrocyclic lactones
4,5 and amines.
6 Moreover, the nucleophilic properties of cesium salts have been used to advantage in substitution reactions.
7Macrocyclic sulfides, including
1,4,8,11-tetrathiacyclotetradecane, are of interest, especially as ligands for transition-metal ions in a variety of different applications.
8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 The methodology described here provides an efficient entry to many such macrocycles, including chiral ones that act as ligands in transition metal-catalyzed coupling reactions.
29
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
bis-Na+ salt of 3,7-dithianonane-1,9-dithiol
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
ether (60-29-7)
sodium chloride (7647-14-5)
1,3-dibromopropane (109-64-8)
nitrogen (7727-37-9)
potassium hydroxide (1310-58-3)
sodium (13966-32-0)
2-chloroethanol (107-07-3)
dichloromethane (75-09-2)
thiourea (62-56-6)
magnesium sulfate (7487-88-9)
N,N-dimethylformamide (68-12-2)
1,3-propanedithiol (109-80-8)
CESIUM (7440-46-2)
1,4,8,11-Tetrathiacyclotetradecane (24194-61-4)
3,7-Dithianonane-1,9-diol (16260-48-3)
3,7-Dithianonane-1,9-dithiol (25676-62-4)
cesium carbonate (29703-01-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved