Org. Synth. 1996, 73, 231
DOI: 10.15227/orgsyn.073.0231
AN IMPROVED PREPARATION OF 3-BROMO-2H-PYRAN-2-ONE: AN AMBIPHILIC DIENE FOR DIELS-ALDER CYCLOADDITIONS
[2H-Pyran-2-one, 3-bromo-]
Submitted by G. H. Posner, K. Afarinkia, and H. Dai
1.
Checked by Alyx-Caroline Guevel and David J. Hart.
1. Procedure
A. 3-Bromo-5,6-dihydro-2H-pyran-2-one. A 1-L, three-necked, round-bottomed flask, equipped with a magnetic stirring bar, a pressure-equalizing addition funnel, a drying tube that contains sodium hydroxide pellets, and a thermometer, is charged with 10.15 g (0.103 mol) of 5,6-dihydro-2H-pyran-2-one (Note 1) and 350 mL of methylene chloride (Note 2). The addition funnel is charged with a solution of 16.7 g (0.105 mol) of bromine (Note 3) in 130 mL of methylene chloride. The bromine solution is added over a period of 4 hr (Note 4) in 10–15-mL portions (Caution! Exothermic reaction. External cooling may be necessary). After the addition of the last portion of bromine, the reaction is analyzed by TLC and NMR (Note 5) and (Note 6). The resulting pale orange solution is stirred for 2 hr until the color has almost faded. The addition funnel is replaced by a rubber septum, and the reaction mixture is cooled by means of an ice bath. Via syringe, 15.0 mL (0.107 mol) of triethylamine is added through the rubber septum over 2 min (Caution! Exothermic reaction). The colorless solution is stirred for 40 min and then the contents of the flask are transferred to a 1-L separatory funnel and washed twice with 150 mL of water. The organic phase is dried over anhydrous sodium sulfate and filtered through a pad of silica gel (Note 7) and the pad rinsed with 700 mL of methylene chloride. The combined filtrates are concentrated at reduced pressure (20 mm) and the last traces of solvent are removed under high vacuum at ambient temperature to afford 16.3 g (89%) of 3-bromo-5,6-dihydro-2H-pyran-2-one as an amber-colored mobile liquid that solidifies when stored overnight at −4°C. This material is sufficiently pure for use in the next step. An analytically pure sample may be prepared by Kugelröhr distillation at 1.0 mm (pot temperature 100°C) (Note 8).
B. 3,5-Dibromo-5,6-dihydro-2H-pyran-2-one. A flame-dried, 1-L, round-bottomed flask, equipped with a magnetic stirring bar and an efficient water-cooled condenser fitted with a nitrogen inlet, is placed under a nitrogen atmosphere and charged with 15.8 g (0.089 mol) of 3-bromo-5,6-dihydro-2H-pyran-2-one, 16.5 g (0.093 mol) of N-bromosuccinimide (Note 1), 0.8 g (3.3 mmol) of benzoyl peroxide (Note 1), and 455 mL of freshly distilled carbon tetrachloride. The reaction flask is immersed in a preheated oil bath (100°C) and the contents are refluxed vigorously for 4.5 hr (Note 9) and (Note 10); the reaction mixture is cooled and allowed to stand at room temperature for 4.5 hr. The precipitate (9.5 g) is collected by suction filtration, the filtrate is concentrated under reduced pressure at 50–60°C (20 mm), and the last traces of solvent are removed under high vacuum (1 mm) at room temperature over 2.5 hr affording 21.5 g (94%) of crude 3,5-dibromo-5,6-dihydro-2H-pyran-2-one as an amber liquid which is sufficiently pure for use in the next step (Note 11). This crude material may be further purified by chromatography (Note 12).
C.
3-Bromo-2H-pyran-2-one. A
1-L, one-necked, round-bottomed flask, equipped with a magnetic stirring bar, and rubber septum through which a
needle-tipped inert gas line is inserted (vented through a
mineral oil bubbler), is charged with
20.3 g of crude 3,5-dibromo-5,6-dihydro-2H-pyran-2-one, as prepared above, and
360 mL of methylene chloride (Note 1). Via syringe,
14.0 mL (0.1 mol) of triethylamine (Note 2) is added over a 5-min period at room temperature. During the addition, the reaction mixture becomes dark brown. After 24 hr, analysis by TLC and NMR indicates the reaction to be complete. The reaction mixture is transferred to a 1-L separatory funnel and washed three times with 140 mL of water. The organic phase is dried over anhydrous
sodium sulfate, filtered and concentrated under reduced pressure at 50–60°C (20 mm). The last traces of solvent and
triethylamine are removed by brief exposure (10 min) to high vacuum at ambient temperature
(Note 13). The residue is purified by chromatography on a 5.0 cm-in-diameter column of 150 g of silica gel packed in
hexane. The crude material is dissolved in a minimum amount of
methylene chloride and applied to the column. Elution with
800 mL of 10% ethyl acetate in hexane followed by
1300 mL of 20% ethyl acetate in hexane, using analytical TLC to monitor fractions, affords in sequence
1.97 g (
14%) of
5-bromo-2-pyrone as a light-brown solid with mp
54–56°C (lit.
2 mp
60–61°C)
(Note 14), and
6.06 g (
43%) of
3-bromo-2-pyrone as a tan solid with mp
59.5–61°C (lit.
3,4 mp
63.5–65°C),
36% yield overall from
5,6-dihydro-2H-pyran-2-one (Note 15) and
(Note 16).
2. Notes
1.
All commercially available reagents were used as received without further purification.
Benzoyl peroxide was 95% pure by iodometric titration.
2.
All common reagents were dried according to recommended procedures
5 and were redistilled prior to use.
3.
The weight of
bromine should be measured by addition to a cooled, pre-weighed
Erlenmeyer flask containing
methylene chloride.
Caution: bromine is toxic and should always be handled in a well-ventilated fumehood.
4.
The rate of addition is determined by the temperature of the reaction mixture and the quality of the starting pyrone. In a duplicate run, starting from redistilled starting material, addition took only 2 hr.
5.
TLC analysis was carried out on precoated plastic silica gel plates (with fluorescent indicator) using
diethyl ether as eluant. With this eluant, the R
f of starting material is 0.50 and the R
f of
3,4-dibromo-3,4,5,6-tetrahydro-2H-pyran-2-one is 0.83. In addition to these two spots, a third spot, R
f = 0.55, corresponding to
3-bromo-5,6-dihydro-2H-pyran-2-one was detected. TLC analysis is not always reliable and it is better to analyze a small aliquot by NMR.
6.
More
bromine may be necessary.
Avoid addition of more bromine if the starting material has already been consumed.
7.
A pad of 100 g of flash silica gel (1.5 cm thick and 11 cm diameter) was used.
8.
Distillation of 1.71 g of crude material afforded
1.50 g of pure
3-bromo-5,6-dihydro-2H-pyran-2-one as a colorless oil which solidifies at −4°C (mp
27–30°C), and which slowly turns yellow upon standing at room temperature. The spectral data for
3-bromo-5,6-dihydro-2H-pyran-2-one are as follows:
1H NMR (400 MHz, CDCl
3) δ: 2.58 (dt, 2 H, J = 6.1, 4.6), 4.49 (t, 2 H, J = 6.1), 7.30 (t, 1 H, J = 4.6);
13C NMR (62.5 MHz, CDCl
3) δ: 26.5, 66.7, 113.3, 146.2, 159.1; IR (neat) cm
−1: 1733, 1615; Mass spectrum (m/z) (EI, 70 eV) 177.9 and 176.9 (M
+, 42). Anal. Calcd for C
5H
5O
2Br: C, 33.91; H, 2.85; Br, 45.16. Found C, 33.99; H, 2.88; Br, 45.08.
9.
Shortly after refluxing begins, the reaction mixture turns dark orange; however, the color dissipates by the end of the reaction period.
10.
Completion of the reaction was checked by TLC analysis
(Note 5). The R
f of the product is 0.60 with
diethyl ether as eluant.
11.
The crude material is contaminated with starting
bromide, small amounts of succinimide and aromatic material, as well as other minor impurities as indicated by
1H NMR.
12.
Chromatography of 1.17 g of this material on 50 g of silica gel with elution by a gradient of
20–50% ethyl acetate in hexane gave
0.90 g (
77%) of
~94% pure 3,5-dibromo-5,6-dihydro-2H-pyran-2-one as a yellow oil that crystallized on standing (mp
60.5–62.5°C; lit.
3 mp
64–65°C). The spectral data for
3,5-dibromo-5,6-dihydro-2H-pyran-2-one are as follows:
1H NMR (400 MHz, CDCl
3) δ: 4.62–4.67 (m, 1 H), 4.76–4.81 (m, 2 H), 7.37 (d, 1 H, J = 5.7). This material was contaminated with
6% of 3-bromo-5,6-dihydro-2H-pyrone as indicated by
1H NMR.
13.
3-Bromo-2-pyrone sublimes in high vacuum at relatively low temperature. Thus, the material should not be subjected to high vacuum for long periods of time.
14.
The spectral data for
5-bromo-2-pyrone are as follows:
1H NMR (400 MHz, CDCl
3) δ: 6.31 (dd, 1 H, J = 9.8, 1.1), 7.38 (dd, 1 H, J = 9.8, 2.7), 7.62 (dd, 1 H, J = 2.7, 1.1);
13C NMR (50 MHz, CDCl
3) δ: 100.7, 117.4, 145.9, 149.6, 159.3.
15.
The spectral data for
3-bromo-2-pyrone are as follows:
1H NMR (400 MHz, CDCl
3) δ: 6.15 (dd, 1 H, J = 6.9, 5.0), 7.51 (dd, 1 H, J = 5.0, 1.9), 7.69 (dd, J = 6.9, 1.9);
13C NMR (50 MHz, CDCl
3) δ: 106.5, 112.6, 144.0, 150.1, 158.0.
16.
The checkers also isolated
0.7 g (
3%) of
3,5-dibromo-2-pyrone as a brown solid, mp
55.5–58.5°C (lit.
2 mp
66–67°C), from the fractions preceding the
5-bromo-2H-pyrone
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure described above allows a more efficient and convenient synthesis of
3-bromo-2-pyrone than previously described in the literature.
4 This procedure avoids making and handling 2-pyrone, a sensitive compound.
6 The major by-product of the reaction is
5-bromo-2-pyrone. We postulate that the formation of this by-product results from prototropic migration in basic medium followed by elimination of
HBr (Scheme 1).
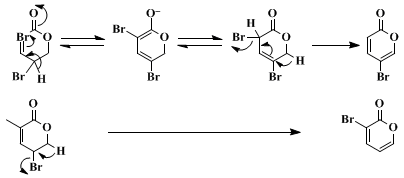
3-Bromo-2-pyrone is not only a valuable precursor for the synthesis of various 3-substituted 2-pyrones,
7 8 but it is also a reactive unsymmetrical diene.
9 10 11 12 13 3-Bromo-2-pyrone undergoes Diels-Alder cycloadditions with a regioselectivity and stereoselectivity that is superior to that of 2-pyrone. Furthermore,
3-bromo-2-pyrone is a chameleon (i.e., ambiphilic) dienophile, undergoing cycloaddition to both electron deficient and electron rich dienophiles. The cycloadducts of bromopyrone with dienophiles are isolable and are useful in the synthesis of diastereomerically pure cyclohexene carboxylates (Scheme 2).
9,10,11,12,13
Z = CH2OSiMe2-t-Bu: (i) Acrolein (10 equiv), 90°C, 96 hr, 70%; then NaBH4, MeOH, 0°C, 15 min (80%); then TSDMSTf, Et3N, CH2Cl2, 30 min (90%); (ii) Bu3SnH, AIBN, benzene, reflux 2 hr (99%); (iii) MeONa, MeOH, 0°C, 92%.
Z = OSiPh2Me: (i) CH2=CHOSiPh2Me (10 equiv), 90°C, 140 hr, 44%; (ii) Bu3SnH, AIBN, benzene, reflux 2 hr (47%); (iii) MeOLi, MeOH, 0°C, 90%.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
TSDMSTf
Et3N
Bu3SnH
AIBN
MeONa
MeOLi
Benzene (71-43-2)
ethyl acetate (141-78-6)
MeOH (67-56-1)
diethyl ether (60-29-7)
Acrolein (107-02-8)
bromide (24959-67-9)
HBr (10035-10-6)
bromine (7726-95-6)
sodium sulfate (7757-82-6)
carbon tetrachloride (56-23-5)
nitrogen (7727-37-9)
methylene chloride,
CH2Cl2 (75-09-2)
benzoyl peroxide (94-36-0)
N-bromosuccinimide (128-08-5)
hexane (110-54-3)
triethylamine (121-44-8)
NaBH4 (16940-66-2)
2-pyrone (504-31-4)
3-Bromo-2H-pyran-2-one,
3-bromo-2-pyrone,
2H-Pyran-2-one, 3-bromo- (19978-32-6)
3-Bromo-5,6-dihydro-2H-pyran-2-one,
3-bromo-5,6-dihydro-2H-pyrone (104184-64-7)
5,6-Dihydro-2H-pyran-2-one (3393-45-1)
3,5-Dibromo-5,6-dihydro-2H-pyran-2-one (56207-18-2)
5-bromo-2-pyrone,
5-bromo-2H-pyrone (19978-33-7)
3,4-dibromo-3,4,5,6-tetrahydro-2H-pyran-2-one
3,5-dibromo-2-pyrone
vitamin D3
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved