Org. Synth. 1995, 72, 74
DOI: 10.15227/orgsyn.072.0074
ASYMMETRIC HYDROGENATION OF ALLYLIC ALCOHOLS USING BINAP-RUTHENIUM COMPLEXES: (S)-(−)-CITRONELLOL
[6-Octen-1-ol, 3,7-dimethyl, (S)-]
Submitted by Hidemasa Takaya
1, Tetsuo Ohta
1, Shin-ichi Inoue
2, Makoto Tokunaga
2, Masato Kitamura
2, and Ryoji Noyori
2.
Checked by Andrew Madin and Larry E. Overman.
1. Procedure
Caution! BINAP-Ru complexes are rapidly oxidized in solution in the presence of air, and all procedures should be carried out under anaerobic conditions using degassed solvents.
A. [(R)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium diacetate, Ru(OCOCH3)2[(R)-BINAP]. A dry, 150-mL Schlenk tube (Note 1) connected to a supply of argon (Note 2) is equipped with a Teflon-coated magnetic stirring bar and a glass stopper. The vessel is charged with benzeneruthenium(II) chloride dimer, [RuCl2(benzene)]2, (800 mg, 1.60 mmol) (Note 3) and (R)-BINAP (1.89 g, 3.04 mmol) (Note 4), evacuated, and then filled with argon. N,N-Dimethylformamide (DMF) (30 mL) (Note 5) is introduced with a hypodermic syringe under the stream of argon. The reddish brown suspension is stirred at 100°C for 10 min (Note 6) and the resulting clear reddish brown solution (Note 7) is cooled. Another dry, 60-mL Schlenk tube is charged with sodium acetate (5.20 g, 63.4 mmol) and methanol (50 mL) (Note 8) and the solution is degassed by three freeze-thaw cycles. It is transferred into the DMF solution of BINAP-Ru(II) complex prepared above via cannula and the solution is stirred at 25°C for 5 min. To the solution are added water (50 mL) and toluene (25 mL) under argon and the resulting two layers are mixed by vigorous stirring. The upper organic layer is transferred into another 200-mL Schlenk tube (Note 9) using a cannula. The aqueous layer is vigorously mixed with toluene (25 mL) and the upper organic layer is again transferred into the Schlenk tube. This procedure is repeated once more. The combined organic layers are washed with four 10-mL portions of water (Note 10). Removal of the solvent at 1 mm and 40°C for 30 min with vigorous stirring (Note 11) and evacuation at 0.1 mm and 25°C for 12 hr gives 2.54–2.67 g of solid Ru(OCOCH3)2 [(R)-BINAP] (99–104% crude yield based on BINAP) (Note 12). It is dissolved in toluene (20–25 mL) with heating. Hexane (80–100 mL) is added very carefully to the solution down the side of the flask to form two layers. The solution is left at 25°C for 12 hr and then at 4°C for 3 days to give solid material. Removal of the mother liquor is followed by washing with hexane (20 mL) and drying under reduced pressure to afford 1.8–2.2 g (71–85% yield) of pure Ru(OCOCH3)2 [(R)-BINAP] as fine yellow needles or powdery crystals; mp 188–190°C dec (Note 13).
B.
(S)-(−)-Citronellol.
Geraniol (12.9 mL, 74 mmol) (Note 14) and
95% methanol (15 mL) (Note 15) are placed in a dry,
80-mL Schlenk tube and the mixture is degassed by five freeze-thaw cycles. Another dry, 80-mL Schlenk tube, equipped with a
rubber septum and a
magnetic stirring bar and filled with
argon, is charged with
Ru(OCOCH3)2 [(R)-BINAP] (45 mg, 53 μmol) (Note 16). The system is evacuated and refilled with
argon three times. By use of a cannula, the solution of
geraniol in
methanol is introduced into the Schlenk tube containing the
ruthenium complex under
argon. The resulting light yellow solution
(Note 17) is then transferred with a cannula into a
100-mL, stainless steel autoclave equipped with a
glass vessel (Note 18) and a magnetic stirring bar by use of a slight positive pressure of
argon. The autoclave is connected to a
hydrogen source (Note 19) using the arrangement shown in
Figure 1, and the air originally present in the
gas-inlet tube is replaced by
hydrogen (Note 20). Valve A is opened and
hydrogen is introduced until pressure gauge B indicates 100 atm. The solution is stirred at 20°C for 8–16 hr
(Note 21). During hydrogenation, the
hydrogen pressure is kept above 90 atm by the occasional introduction of
hydrogen from the
cylinder (Note 22). When consumption of
hydrogen ceases, the gas-inlet tube is disconnected. Excess
hydrogen is carefully released by opening valve A and then the apparatus is disassembled. The yellowish brown contents
(Note 23) are transferred to a 50-mL flask and the solvent is removed by a
rotary evaporator. The residue
(Note 24) is distilled under reduced pressure to give
10.7–11.2 g (
93–97% yield) of
(S)-(−)-citronellol in 98% ee
(Note 25) and
(Note 26); bp
58–62°C/0.01 mm.
Figure 1
2. Notes
1.
Before use, all apparatus is dried overnight in a 120°C
oven.
2.
Argon (99.998%) is purified by passing through a BASF catalyst RC-11 column at 80°C and then through 4 Å molecular sieves.
3.
[RuCl2(benzene)]2, available from Aldrich Chemical Company, Inc., is used without purification.
4.
BINAP [2,2'-bis(diphenylphosphino)-1,1'-binaphthyl] is commercially available or can be prepared according to a literature procedure.
3 The diphosphine is slowly oxidized in air to give the corresponding mono- and diphosphine oxides that can be removed by column chromatography (silica gel,
benzene) under an inert atmosphere.
5.
Guaranteed grade DMF, available from NACALAI TESQUE, Inc., is distilled over 4 Å molecular sieves under
argon before use and stored in a
100-mL Schlenk tube. It is degassed by three freeze-thaw cycles.
6.
Reaction at a higher temperature for a longer period leads to formation of the
ruthenium carbonyl complex [IR(KBr) 1964 cm
−1].
7.
The solution may be a crude mixture of cationic BINAP-Ru(II) complexes such as [RuCl(BINAP)(DMF)
3]Cl and [Ru(BINAP)(DMF)
4]Cl
2. Physical properties include conductivity, 27 Scm
2/mol (DMF);
31P NMR (4:1 DMF-CDCl
3) δ: 60.6 (d, J = 46), 61.4 (d, J = 46), 61.8 (s). The (R)-BINAP-Ru(II) complex obtained by removal of the solvent can catalyze hydrogenation of
geraniol (
98.7% pure commercial geraniol containing 1.3% of nerol, distilled from 4 Å molecular sieves). However, in addition to
(S)-citronellol in 95–96% ee,
dihydrocitronellol, an overreduction product, is obtained in
3–7% yield (1.1 M substrate in
95% aqueous methanol, 1.7 mM catalyst, 100 atm of H
2, 20°C, 8 hr).
8.
Guaranteed grade methanol is distilled under
argon.
9.
The diameter is about 4 cm.
10.
The extraction procedure must be carried out under an
argon atmosphere.
11.
Under reduced pressure, the solution sometimes foams. This can be avoided by heating the top part of the Schlenk tube with a hot air gun.
12.
Crude BINAP-Ru complex with consistent spectral characteristics can be used for hydrogenation of
geraniol (
98.7% pure commercial geraniol containing 1.3% of nerol, distilled from 4 Å molecular sieves, 4.7 M substrate in
95% aqueous methanol, 2.8 mM Ru(OCOCH
3)
2 [(R)-BINAP], 100 atm of H
2, 20°C, 8 hr), to give
(S)-citronellol in 96% ee,
97% isolated yield.
13.
The product has the following spectral properties:
1H NMR (CDCl
3) δ: 1.80 (s, 2 OCOCH
3), 6.47–7.84 (m, aromatic protons);
31P NMR (CDCl
3) δ: 65.13 (s);
13C NMR (CDCl
3) δ:23.50, 125.2–138.3, 188.1; IR (CH
2Cl
2) cm
−1: 1452, 1518. An analytical sample is prepared by drying at 110°C and 0.01 mm for 12 hr: Calcd for C
48H
38O
4P
2Ru: C, 68.5; H, 4.6. Found: C, 68.4; H, 4.5.
14.
Pure
geraniol is obtained by fractional distillation using a
1000 theoretical plate column. The checkers obtained
geraniol, > 99.5% purity by GC analysis, from Fluka Chemical Company and used it directly.
15.
The
95% methanol is prepared by mixing distilled, guaranteed
methanol (95 mL) and water (5 mL). If
absolute methanol or 90% aqueous methanol is used as solvent, somewhat longer reaction times are needed.
16.
The complex can be stored under
argon without noticeable loss of catalytic activity. It is weighed under an
argon atmosphere.
17.
The ruthenium complex is moderately soluble in
methanol. Ultrasonic stirring is employed for complete solution.
18.
A glass vessel is used for keeping the reaction mixture away from the stainless steel wall. The reaction system is evacuated and filled with
argon three times before use.
19.
The purity of
hydrogen (Nippon Sanso Co.) used by the submitters is 99.99999%. The checkers used
hydrogen of 99.99% purity.
20.
The gas-inlet tube is attached to the autoclave and then the main valve of the cylinder is opened. After closing the main valve of the cylinder, the connector of the gas-inlet tube is loosened to release
hydrogen pressure and tightened immediately. This procedure is repeated five times.
21.
To maintain an internal temperature of 20°C the checkers placed the autoclave in a 18°C bath. Reactions terminated after 8 hr by the checkers showed the presence of unreacted
geraniol (2–4%).
22.
Enantioselectivity is very dependent on
hydrogen pressure. Optical purities of
citronellol products are 70% and 95% at 4 and 30 atm, respectively. Thus,
hydrogen pressure greater than 90 atm is required for high optical yields.
23.
In air, the color gradually changes to dark green.
24.
Gas chromatographic analysis indicates that this consists of
97–99% of
citronellol and
1–3% of
dihydrocitronellol; column, SHIMADZU HiCap-CBP20, 25 m × 0.2-mm fused silica; column temperature, 140°C; injection temperature 160°C;
helium pressure as carrier gas, 1.0 kg/cm
2; t
R of
geraniol,
citronellol, and
dihydrocitronellol are 16.2, 13.7, and 8.6 min, respectively.
25.
The product has the following spectral properties;
1H NMR (400 MHz, CDCl
3) δ: 0.89 (d, J = 6.6, CH
2CH(CH
3)CH
2), 1.18 (m, CH
2CHHCH(CH
3)CH
2), 1.36 (m, CH
2CHHCH(CH
3)CHH), 1.57 (m, CH
2CH(CH
3)CHH), 1.59 (s, (CH
3)
2C=CH), 1.67 (s, (CH
3)
2C=CH), 1.71 (s, CH
2CH
2OH), 1.97 (m, (CH
3)
2C=CHCH
2), 3.66 (m, CH
2CH
2OH), 5.08 (t, J = 6.96, (CH
3)
2C-CH),
[α]29D −4.5° to −4.7° (neat) [lit.
4 [α]20D −4.76° (neat)]. The submitters determined the enantiomeric excess by HPLC analysis of the diastereomeric amide prepared by condensation of
citronellic acid (Note 27), obtained by the Jones oxidation of the alcohol, and
(R)-1-(1-naphthyl)ethylamine (Note 28). HPLC analysis of this amide (column, TOYO SODA SILICA-60, 4.6 × 500 mm, a 3:7
ether-hexane mixture; flow rate 1 mL/min; detection 254 nm light) showed two peaks with t
R at 33.0 and 36.0 min in 1:99 ratio assignable to the R,R- and R,S-diastereomers, respectively.
26.
With a commercially available 98.7:1.3 mixture of
geraniol and
nerol as substrate,
(S)-citronellol in 96% ee is obtained. See also
(Note 7).
27.
In a
100-mL, round-bottomed flask equipped with a magnetic stirring bar are placed
β-citronellol (1.63 g, 10.4 mmol) and
acetone (25 mL). To this is slowly added
Jones reagent [Na2Cr2O7 (0.73 M in H2O)/H2SO4 (concn = 1/4.2 v/v)] at 0–5°C until the orange color remains unchanged. To this mixture is added
2-propanol (5 mL) to decompose excess Jones reagent. After the orange color disappears, insoluble material is filtered off and the solvent is removed under reduced pressure. The residue is dissolved in
ether (10 mL) and the solution is washed with
brine (10 mL). The water layer is extracted with
ether and the combined organic layers are extracted with
1 N aqueous sodium hydroxide (50 mL) at 0°C. The alkaline aqueous layer is acidified and extracted with three,
20-mL portions of ether at 0°C. The combined
ether layers are dried over anhydrous
magnesium sulfate and the solvent is evaporated. To the residue is added a small amount of
benzene; the
benzene is then removed under reduced pressure to give
977 mg (
55% yield) of
citronellic acid.
28.
In a
50-mL, round-bottomed flask, equipped with a rubber septum and a magnetic stirring bar and filled with
argon, are placed
citronellic acid (112 mg, 0.658 mmol) and a solution of
(R)-1-(1-naphthyl)ethylamine (140 mg, 0.818 mmol) (Note 29) in dry DMF (4 mL) (Note 5). To this mixture in an
ice bath are added a solution of
diethyl cyanophosphonate (224 mg, 1.37 mmol) (Note 30) in dry DMF (2 mL) and then dry
triethylamine (0.1 mL). The mixture is stirred and the reaction is followed by TLC. When the carboxylic acid has been consumed, which takes approximately 2 hr at room temperature, the reaction mixture is dissolved in a mixture of
benzene (5 mL) and
ethyl acetate (5 mL). The mixture is washed successively with cold
5% hydrochloric acid, ice-water, saturated
sodium hydrogen carbonate solution, and brine, dried over anhydrous
sodium sulfate, and finally concentrated under vacuum to give 207 mg (97% yield) of the amide.
29.
(R)-(+)-1-(1-Naphthyl)ethylamine is purchased from Aldrich Chemical Company, Inc. The reagent is purified by recrystallization of its tartaric acid salt three times from
94% aqueous methanol followed by treatment with base and distillation of the free amine.
30.
Commercial grade diethyl cyanophosphonate is distilled under
argon before use.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure for the synthesis of the title compound is a representative example of asymmetric hydrogenation in the presence of
BINAP-Ru(II) diacetate.
5 The method is based on the synthesis of BINAP-Ru(II) dicarboxylate complexes
6 and enantioselective hydrogenation of
geraniol.
7 The present method provides the first practical means for asymmetric synthesis of
(S)- and (R)-citronellol.
(S)-(−)-Citronellol of optical purity up to 92% can be obtained in a limited quantity from rose oil. A microbiological reduction of
geraniol was reported to give enantiomerically pure
(R)-(+)-citronellol.
8Nerol can also be used as a substrate. The stereochemical outcome is shown in Scheme 1, which indicates that the BINAP-Ru species differentiates the C(2) enantiofaces. The C(6)-C(7) double bonds are left intact. Thus, both R and S enantiomers are accessible by either variation of allylic olefin geometry or choice of handedness of the catalysts.
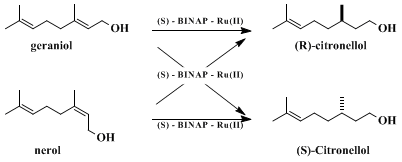
The complex formulated as
Ru(OCOCF3)2 (BINAP), that is prepared from
Ru(OCOCH3)2 (BINAP) and excess
trifluoroacetic acid, shows high catalytic activity (S/C = 50000, 20°C, 100 atm, 96% ee). The cationic complex
[RuI(BINAP)(p-cymene)]I9 also acts as an efficient catalyst (96% ee). In the presence of the catalyst system derived from
Ru(OCOCH3)2(BINAP) and 1 equiv of aqueous
perchloric acid, hydrogenation proceeds very rapidly (S/C = 2000, completion after 15 min), but results in low enantioselectivity (94% ee).
ToIBINAP10 [2,2'-bis(di-p-tolylphosphino)-1,1'-binaphthyl] can also be used. Hydrogenation of
geraniol using
Ru2Cl4 [(R)-BINAP]2[N(C2H5)3]11 as catalyst (
6:1 ethanol-dichloromethane, H
2, 40 atm, 24°C, 90 hr) gives
(S)-citronellol in
47% yield and 93% ee (92% conversion) in addition to
dihydrocitronellol (
40% yield) (see also
(Note 7)).
This procedure has been successfully applied to the asymmetric synthesis of
(3R,7R)-3,7,11-trimethyldodecanol, a versatile intermediate for synthesis of α-tocopherol (vitamin E). Hydrogenation of
homogeraniol also proceeds smoothly to give
4,8-dimethylnon-7-enol in 92% ee in
96% yield. Hydrogenation of racemic allylic secondary alcohols in the presence of
BINAP-Ru(II) diacetate brings about a high level of kinetic enantiomer selection.
12 This method provides a practical route to
(R)-4-hydroxy-2-cyclopentenone, an important building block for the three-component prostaglandin synthesis.
13 A useful intermediate for synthesis of 1β-methylcarbapenems can be prepared by the hydrogenation of a chiral
allylic alcohol.
14BINAP-ruthenium dicarboxylate complexes are also efficient catalysts for asymmetric hydrogenation of enamides, α,β– and β,γ-unsaturated carboxylic acids, α-amino ketones, and α-acylaminoacrylic acids.
5,15A detailed procedure for the asymmetric hydrogenation of β-ketoesters using a BINAP-ruthenium complex may be found on
p. 589.
16This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
Homogeraniol
(S)-(−)-CITRONELLOL
[(R)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium diacetate, Ru(OCOCH3)2[(R)-BINAP]
benzeneruthenium(II) chloride dimer
BINAP-Ru(II) complex
Ru(OCOCH3)2 [(R)-BINAP]
ruthenium complex
BINAP [2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]
ruthenium carbonyl complex
BINAP-Ru(II) diacetate
(S)- and (R)-citronellol
Ru(OCOCF3)2 (BINAP)
Ru(OCOCH3)2 (BINAP)
Ru(OCOCH3)2(BINAP)
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
ethyl acetate (141-78-6)
methanol (67-56-1)
ether (60-29-7)
sodium acetate (127-09-3)
hydrogen (1333-74-0)
sodium hydroxide (1310-73-2)
sodium hydrogen carbonate (144-55-8)
allylic alcohol (107-18-6)
sodium sulfate (7757-82-6)
acetone (67-64-1)
toluene (108-88-3)
2-propanol (67-63-0)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
N,N-dimethylformamide,
DMF (68-12-2)
hexane (110-54-3)
geraniol (106-24-1)
triethylamine (121-44-8)
argon (7440-37-1)
perchloric acid (7601-90-3)
trifluoroacetic acid (76-05-1)
helium (7440-59-7)
nerol (106-25-2)
citronellol,
dihydrocitronellol,
β-citronellol (106-22-9)
(R)-4-hydroxy-2-cyclopentenone
(S)-Citronellol,
6-Octen-1-ol, 3,7-dimethyl, (S)- (7540-51-4)
citronellic acid (502-47-6)
(R)-1-(1-naphthyl)ethylamine,
(R)-(+)-1-(1-Naphthyl)ethylamine (42882-31-5)
diethyl cyanophosphonate (2942-58-7)
(R)-(+)-citronellol
(3R,7R)-3,7,11-trimethyldodecanol
4,8-dimethylnon-7-enol (163182-73-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved