Org. Synth. 1993, 71, 158
DOI: 10.15227/orgsyn.071.0158
BENZOANNELATION OF KETONES: 3,4-CYCLODODECENO-1-METHYLBENZENE
[Benzocyclododecene, 5,6,7,8,9,10,11,12,13,14-decahydro-2-methyl-]
Submitted by Marcus A. Tius and G. S. Kamali Kannangara
1.
Checked by Annapoorna Akella and James D. White.
1. Procedure
A. 1-(2-Methylallyl)-2-(trimethylsiloxy)methylenecyclododecanol. A dry, 2-L, three-necked, round-bottomed flask connected to a nitrogen bubbler and equipped with a mechanical stirrer with ground glass shaft and bearing a 100-mL pressure-equalizing dropping funnel and a septum inlet is charged with 45.4 g (1.89 mol) of magnesium turnings (Note 1) and flushed for 5 min with nitrogen. Anhydrous ether, 450 mL, (Note 2) is added and the flask is cooled to 0°C in an ice bath. Methallyl chloride, 64.6 mL (0.65 mol, (Note 3)) is added dropwise from the addition funnel to the stirred magnesium turnings during 45 min. (Caution! The reaction is exothermic and care must be exercised to add the methallyl chloride at a moderate rate with adequate stirring and cooling of the reaction mixture). During this time the reaction mixture turns to a gray heterogeneous slurry. Stirring is continued at 0°C for 1.5 hr and at 22°C for 1.5 hr.
In a separate, dry, 1-L, two-necked, round-bottomed flask fitted to a nitrogen bubbler and equipped with a magnetic stirring bar and a septum inlet is added a solution of 12.6 g (60.0 mmol) of 2-(hydroxymethylene)cyclododecanone (Note 4) in 500 mL of anhydrous ether. The stirred ethereal solution of the hydroxymethylene ketone is treated at 22°C with 33 mL of a freshly prepared mixture (1/1, v/v) of chlorotrimethylsilane and triethylamine (Note 5). An immediate reaction takes place with deposition of a white precipitate. The mixture is stirred thoroughly at 22°C for 15 min to insure complete conversion to the silyl enol ether.
The heterogeneous mixture of the silyl enol ether and triethylamine hydrochloride is transferred to the solution of the Grignard reagent at 0°C by means of a large-bore cannula (Note 6) during 15 min. The efficient transfer of the silyl enol ether is accomplished with the aid of nitrogen pressure. The flask containing the silyl enol ether is rinsed with 50 mL of ether that is transferred to the Grignard solution. Stirring at 0°C is continued for 15 min. The reaction is then quenched by slow addition of saturated aqueous sodium chloride solution until the reaction mixture becomes clear. (Caution! The septa are removed from the flask in order to vent the pressure). The solution is allowed to warm to 22°C and the ether layer is decanted from the magnesium salt and the unreacted magnesium. The residue is diluted with 100 mL of saturated aqueous sodium chloride solution and extracted with ether (3 × 50 mL). The combined ether extracts are washed with brine (2 × 50 mL), dried over anhydrous magnesium sulfate and concentrated at reduced pressure. The product that is obtained is used in the next step without purification.
B. 3,4-Cyclododeceno-1-methylbenzene. A 1-L, two-necked, round-bottomed flask equipped with a reflux condenser, septum inlet, and a magnetic stirring bar is fitted to a nitrogen bubbler. The flask is charged with 200 mL of toluene (Note 7) and 4.6 g (24 mmol) of p-toluenesulfonic acid monohydrate (Aldrich Chemical Company, Inc.). The solution is warmed to 80°C in a heating mantle. Tertiary alcohol 1 from the preceding step is dissolved in 150 mL of toluene and transferred to the flask by cannula. The progress of the reaction can be monitored by silica gel TLC eluting with 20% ethyl acetate in hexane (Note 8). After 3 hr the reaction mixture is cooled to 22°C and washed with saturated aqueous sodium bicarbonate (2 × 100 mL). The aqueous phase is extracted with ether (4 × 100 mL) and the combined organic extracts are dried over anhydrous magnesium sulfate. The solvents are removed under reduced pressure and the residue is purified by flash column chromatography on silica gel, eluting with hexane. The benzoannelated product is obtained as a pale yellow oil in 86% overall yield (12 g; (Note 9)).
2. Notes
1.
Magnesium turnings (98%) from Aldrich Chemical Company, Inc. were used after drying in a
beaker at 110°C overnight. The Grignard reaction took place without need of an initiator.
2.
Reagent grade ether was dried by distillation under
argon from a purple solution of
sodium benzophenone ketyl.
3.
Methallyl chloride (obtained from Aldrich Chemical Company, Inc.) was distilled (bp
71–72°C) from
phosphorus pentoxide prior to use.
4.
See, Ainsworth, C.
Org. Synth., Coll. Vol. IV 1963, 536 and Woodward, R. B.; Pachter, I. J.; Scheinbaum, M. L.
Org. Synth., Coll. Vol. VI 1988, 590. The following modified procedure was used: To a
1-L, three-necked, round-bottomed flask equipped with a septum inlet, mechanical stirrer,
nitrogen inlet, and pressure-equalizing dropping funnel was added
3.0 g (92 mmol) of sodium hydride (obtained from Aldrich Chemical Company, Inc.). The mineral oil was removed from the
sodium hydride by washing with
hexane.
Ether was added (250 mL) and the reaction mixture cooled to 0°C. A mixture of
14 g (77 mmol) of cyclododecanone (obtained from Aldrich Chemical Company, Inc.) and
6.8 mL (85 mmol) of ethyl formate (obtained from Aldrich Chemical Company, Inc., and distilled from
phosphorus pentoxide) in
75 mL of ether was added through the
dropping funnel over 45 min. Next,
4 mL of methanol was cautiously added. After 30 min the reaction became heterogeneous. Further addition of
4 mL of methanol allowed stirring to take place. The reaction mixture was then stirred for 4 hr at 0°C. The cooling bath was removed and stirring was continued for 8 hr at 23°C. The reaction was worked up by collecting the light yellow solid and dissolving it in 150 mL of water. The aqueous layer was washed once with ether to remove unreacted ketone and residual mineral oil. Careful acidification with
1 N hydrochloric acid to pH 6 was followed by extraction with
ether (6 × 50 mL). The
ether extracts were washed with
brine (2 × 50 mL), dried (MgSO
4) and concentrated to produce
11.5 g of the hydroxymethylene ketone, which was used in the next step without purification. The spectrum was as follows:
1H NMR (300 MHz, CDCl
3) δ: 1.30–1.39 (m, 12 H), 1.50–1.58 (br m, 2 H), 1.78–1.80 (br m, 2 H), 2.26 (t, 2 H, J = 6.9), 2.35 (t, 2 H, J = 7.5), 8.58 (d, 1 H, J = 3.6).
5.
Chlorotrimethylsilane (obtained from Aldrich Chemical Company, Inc.) and
triethylamine (obtained from Aldrich Chemical Company, Inc.) were mixed in equal volumes in dry, stoppered tubes. These were centrifuged briefly and the supernatant was transferred through a septum by syringe.
6.
A suitable cannula was made by filing the ends of a
45-cm long aluminum tube of 2-mm internal diameter to points.
7.
Reagent grade toluene (obtained from Fisher Scientific Company) was degassed with a
nitrogen stream.
8.
The reaction was most easily monitored by noting the disappearance of the highly uv absorbing unsaturated aldehyde intermediate at R
f = 0.41 (
20% ethyl acetate in hexane eluant).
9.
The physical properties are as follows:
1H NMR (300 MHz, CDCl
3) δ: 1.39–1.44 (m, 8 H), 1.51–1.54 (m, 4 H), 1.65–1.76 (m, 4 H), 2.29 (s, 3 H), 2.62 (t, 4 H, J = 7.5), 6.93–7.09 (m, 3 H);
13C NMR (125 MHz, CDCl
3) δ: 20.95, 22.84, 22.85, 25.54 (two peaks overlap), 26.25, 26.30, 28.93, 29.29, 29.89, 29.98, 126.52, 129.45, 130.21, 134.91, 137.86, 140.74; IR (neat) cm
−1: 2940, 2880, 1510, 1475, 1450, 830, 810; mass spectrum m/e 230 (M
+, 82%), 215 (4%), 173 (8%), 159 (20%), 145 (50%), 119 (100%), 105 (42%), 91 (20%), 40 (37%).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
This preparation describes a highly practical and efficient method for the benzoannelation of ketones. The classical approach to the synthesis of aromatic compounds has been to start with a commercially available aromatic compound and make use of traditional substitution reactions in order to introduce appendages and functionality. This strategy has served well, but it is limiting for multistep organic synthesis because it normally requires that the aromatic substitution chemistry be carried out at the very beginning of a sequence. The method described here provides an alternative strategy in which a non-aromatic compound can serve as a precursor to an aromatic molecule.
The present method is successful with a wide variety of ketones (see Table). Cyclic ketones (entries 1–4, 8) produce benzoannelated products in excellent overall yields. There is no need to purify the intermediate; both the nucleophilic addition of
methallylmagnesium chloride and the aromatic cyclization take place cleanly. Acyclic ketones (entries 5–7) also provide high yields of benzoannelated product. Aromatic ketones are particularly interesting substrates for this reaction since they provide substituted biphenyls, which are potentially useful materials for liquid crystal synthesis and whose preparation through classical methodology is often not straightforward. The conditions for the cationic cyclization step can be modified to accommodate acid-sensitive functionality. For example, cyclization of
3 to
4, the latter a precursor for 3-methyl-8,14-dehydromorphinan, was accomplished in 77% yield by treatment of
3 at 23°C for 12 hr with
pyridinium tosylate in
benzene.
2 This is an extraordinarily mild procedure for an aromatic annelation. The method is not limited to the preparation of methyl-substituted aromatics. By using
benzylmagnesium bromide instead of methallyl Grignard reagent, a
naphthalene can be appended onto the ketone.
3 Similarly, phenanthrenes
3 and m-terphenyls
4 can be obtained conveniently and in high yield. In the absence of a cation-stabilizing group at C-2 of the Grignard reagent, the yield for cyclization is diminished.
5 A similar effect has been noted in related work.
6 Unsubstituted, benzoannelated products are nevertheless accessible from cyclization of the adducts of
[2-(trimethylsilyl)-2-propenyl]magnesium chloride.
5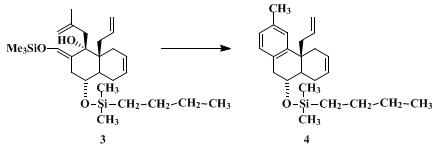
3 4
TABLE
BENZOANNELATION OF KETONES
|
|
Entry
|
2-Hydroxymethylene ketone
|
Product
|
Overall yield (%)
|
|
|
1
|
|
|
52a
|
|
2
|
|
|
76b
|
|
3
|
|
|
79c
|
|
4
|
|
|
74d
|
|
5
|
|
|
66e
|
|
6
|
|
|
89f
|
|
7
|
|
|
65g
|
|
8
|
|
|
86
|
|
Several related methods for benzoannelation have been reported.
7 8 9 10 Most provide aromatic sulfides or phenols that require additional manipulation. The present method provides convenient and highly efficient access to structurally diverse benzoannelated products. The ease of the reactions, the high yields, and the convenience recommend its use.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
sodium benzophenone ketyl
3,4-Cyclododeceno-1-methylbenzene
Tertiary alcohol
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
ethyl acetate (141-78-6)
methanol (67-56-1)
ether (60-29-7)
sodium bicarbonate (144-55-8)
magnesium,
magnesium turnings (7439-95-4)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
toluene (108-88-3)
Naphthalene (91-20-3)
ethyl formate (109-94-4)
Triethylamine hydrochloride (554-68-7)
magnesium sulfate (7487-88-9)
benzylmagnesium bromide (1589-82-8)
sodium hydride (7646-69-7)
hexane (110-54-3)
triethylamine (121-44-8)
argon (7440-37-1)
methallyl chloride (563-47-3)
cyclododecanone (830-13-7)
CHLOROTRIMETHYLSILANE (75-77-4)
phosphorus pentoxide (1314-56-3)
p-toluenesulfonic acid monohydrate (6192-52-5)
Benzocyclododecene, 5,6,7,8,9,10,11,12,13,14-decahydro-2-methyl- (81857-28-5)
1-(2-Methylallyl)-2-(trimethylsiloxy)methylenecyclododecanol (155727-13-2)
2-(hydroxymethylene)cyclododecanone (949-07-5)
methallylmagnesium chloride
pyridinium tosylate
[2-(trimethylsilyl)-2-propenyl]magnesium chloride
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved