Org. Synth. 1995, 72, 154
DOI: 10.15227/orgsyn.072.0154
GENERATION OF N,N-DIETHYLCARBAMOYLLITHIUM VIA LITHIUM-TELLURIUM EXCHANGE AND ITS REACTION WITH 3-PHENYLPROPANAL: N,N-DIETHYL-2-HYDROXY-4-PHENYLBUTANAMIDE
[Benzenebutanamide, N,N-diethyl-α-hydroxy-]
Submitted by Nobuaki Kambe, Toru Inoue, and Noboru Sonoda
1.
Checked by Thomas G. Gant and Albert I. Meyers.
1. Procedure
Finely ground elemental tellurium (13.40 g, 105 mmol) (Note 1) and tetrahydrofuran (THF, 400 mL) (Note 2) are placed under nitrogen (N2) in a flame-dried, 1-L, three-necked, round-bottomed flask equipped with a N2 inlet, 100-mL pressure-equalizing dropping funnel, and a rubber septum. Into the mixture is added ca. 65.5 mL (1.6 N in hexane, ca. 105 mmol) of butyllithium (Note 3) dropwise with a syringe through the septum with stirring at room temperature until the mixture becomes a pale yellow homogeneous solution (Note 4). The solution is stirred for 10 min, then cooled to −78°C. Diethylcarbamoyl chloride (12.7 mL, 13.56 g, 100 mmol) (Note 5) is injected through the septum with a syringe, and the solution is stirred at −78°C for 10 min. The cold bath is removed and the solution is allowed to warm to room temperature, then stirred for 1 hr. It is cooled again to −78°C, and 62.5 mL (1.6 N, 100 mmol) of butyllithium (Note 3) is injected over a 3-min period. After the solution is stirred at −78°C for 5 min, 13.42 g (100 mmol) of 3-phenylpropanal (Note 6) in 50 mL of THF is added from the dropping funnel over a 5-min period. The mixture is stirred for 10 min, warmed to room temperature, and stirred for 1 hr. The mixture is poured into 200 mL of a saturated ammonium chloride solution and extracted with diethyl ether (Et2O) three times, 30 mL each. The combined organic layers are dried over anhydrous magnesium sulfate, and concentrated on a rotary evaporator at ca. 5 mm. The residual yellowish brown oil is chromatographed (silica gel, 50 mm × 25 cm) (Note 7). The first, pale yellow fraction eluted with ca. 400 mL of hexane contains dibutyl telluride (23.62 g, 93% based on tellurium used) (Note 8). The next 500-mL fraction (eluted with hexane/Et2O, 10/1) contains by-products (Note 9). The desired product is eluted in the third fraction (ca. 500 mL of Et2O) that is concentrated on a rotary evaporator and distilled to afford 19.35 g of N,N-diethyl-2-hydroxy-4-phenylbutanamide as a pale yellow viscous oil (bp 155–156°C at 0.6 mm, 82%) (Note 10),(Note 11),(Note 12).
2. Notes
1.
The submitters used
tellurium pieces from Aldrich Chemical Company, Inc., that were ground with a
mortar and pestle just before use. The checkers found that direct use of
tellurium powder (–60 mesh, Aldrich Chemical Company, Inc.) gave similar results.
2.
THF purchased from Wako Pure Chemical Industries, Ltd. (Japan) was used after distillation from Drynap. Drynap is a registered trademark of an alloy of
sodium (above 8 wt%) and lead (90 wt%) produced by Dojin Chemical Research Center, Ltd. (Japan). Although Drynap is not essential and any procedure can be used for drying
THF provided that the
THF is sufficiently dry for the usual reactions of organolithium compounds, the submitters often use this drying reagent when a large amount of solvent is needed since it is mild, much safer than
sodium, and is easy to handle.
3.
The submitters used
butyllithium (1.6 M in hexane) from Nacalai Tesque Company, Ltd. (Japan). The checkers used
butyllithium (1.6 M in hexane) from Aldrich Chemical Company, Inc.4.
The reaction is slightly exothermic. At this stage,
lithium butanetelluroate (BuTeLi) is formed. (The checkers noted a deep red color as the BuLi was added.)
BuTeLi should be prepared slightly in excess to the carbamoyl chloride. The subsequent step produces carbamoyllithium that may react with any excess carbamoyl chloride to give an undesired oxamide by-product.
5.
The submitters used
diethylcarbamoyl chloride from Aldrich Chemical Company, Inc., after fractional distillation. The checkers used the reagent without further purification with similar results.
6.
The submitters used
3-phenylpropanal from Wako Pure Chemical Industries, Ltd., after distillation. The checkers used reagent obtained from Lancaster Synthesis without further purification with similar results.
7.
The submitters used
silica gel (mesh 100–200, BW-820 MH) from Fuji Davison Chemical, Ltd. (Japan). The checkers used radial chromatography (
silica gel 60 PF 254 with gypsum, EM Science) with
hexane/
ethyl acetate (4/1) as eluent for small scale reactions. The checkers used flash chromatography (
240 g of silica gel, grade 633, 47 × 61 microns, Davison Chemical) with
hexane (1.5 L),
hexane/ether (10/1, 1.5 L), and
ether (1.5 L) as eluent for large scale reactions (100 mmol).
8.
Elemental
tellurium can be recovered from
dibutyl telluride by the following procedure. Under
nitrogen,
naphthalene (4.0 g) and dry
THF (100 mL) are placed in a flame-dried,
200-mL, three-necked, round-bottomed flask equipped with a N
2 inlet,
reflux condenser, and rubber septum.
Sodium pieces (4.28 g, 186 mmol) are added to the solution slowly with stirring. After the solution turns deep blue,
4.50 g (18.6 mmol) of dibutyl telluride is added over a 3-min period with a syringe at room temperature. The rubber septum is replaced with a
glass stopper and the mixture is refluxed for 2 days. The mixture is cooled to room temperature and
ethanol is added very slowly until the
sodium completely disappears. Air is bubbled into the solution with stirring for one day. The black solids that are obtained by filtration are washed well with
Et2O and dried (0.2 mm, 250°C, 4 hr) to give
2.22 g of elemental
tellurium (
93%, based on
dibutyl telluride used). These conditions have not been optimized.
9.
This fraction contains
3-phenylpropanal and
1-phenylheptan-3-ol that is formed by the addition of
butyllithium to
3-phenylpropanal.
10.
The physical properties are as follows:
1H NMR (270 MHz, CDCl
3) δ: 1.09 (t, 3 H, J = 6.9), 1.10 (t, 3 H, J = 6.9), 1.72–1.94 (m, 2 H), 2.72–2.87 (m, 2 H), 2.98–3.13 (m, 2 H), 3.25 (dq, 1 H, J = 13.8, 6.9), 3.52 (dq, 1 H, J = 13.8, 6.9), 3.87 (d, 1 H, J = 7.8), 4.24 (ddd, 1 H, J = 7.8, 7.8, 2.9), 7.16–7.37 (m, 5 H);
13C NMR (68 MHz, CDCl
3) δ: 12.8, 14.0, 31.3, 37.4, 40.2, 40.8, 67.0, 120.0, 128.5, 128.6, 141.4, 173.6; IR (NaCl, neat) cm
−1: 3408, 1636. Anal. Calcd for C
14H
21NO
2: C, 71.45; H, 9.00; N, 5.95. Found: C, 71.17; H, 9.08; N, 6.01.
11.
Glassware is cleaned by soaking it overnight in a concd
nitric acid bath.
12.
When
methyl benzoate was used as an electrophile instead of
3-phenylpropanal,
N,N-diethylphenylglyoxylamide [Et2NC(O)C(O)Ph] was obtained in a similar manner as a pale yellow viscous oil (bp
118–120°C at 0.2 mm,
13.40 g, 65 mmol,
65%),
1H NMR (270 MHz, CDCl
3) δ: 1.15 (t, 3 H, J = 7.2), 1.29 (t, 3 H, J = 7.2), 3.24 (q, 2 H, J = 7.2), 3.56 (q, 2 H, J = 7.2), 7.50 (t, 2 H, J = 7.3), 7.63 (t, 1 H, J = 7.3), 7.94 (d, 2 H, J = 7.3); IR (NaCl, neat) cm
−1: 1681, 1636, 1448, 1232, 721.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Organic tellurium compounds react readily with organolithium reagents to give the thermodynamically more stable organolithium compounds via tellurium ate complexes. The
lithium-tellurium exchange reaction proceeds quite rapidly at low temperatures, and can be used for the generation of a variety of organolithium compounds,
2,3,4,5 including very reactive umpolung species, such as acyl-,
3 aroyl-,
3 and carbamoyllithiums.
4 Among these, acyl- and aroyllithiums are kinetically very unstable, even at low temperatures, and dimerize quickly unless a suitable electrophile is present when generated. In contrast N,N-dialkylcarbamoyllithiums are potentially useful synthetically since they are stable in THF at −78°C and can be efficiently trapped with various electrophiles.
4Carbamoyllithiums are attractive umpolung species that enable the straightforward introduction of carbamoyl moieties into organic molecules as nucleophiles. Although several methods have been reported for the generation of carbamoyllithiums, such as abstraction of the
formyl hydrogen,
6 insertion of
carbon monoxide into lithium amides
7 or lithium diaminocuprates,
8 and transmetalation of a
biscarbamoylmercury,
9 these procedures have some disadvantages. Very few examples of
hydrogen abstraction are reported, and satisfactory yields of the addition products are obtained only in the case of
N,N-diisopropylcarbamoyllithium, or when the reaction is conducted in the presence of an electrophile. Reaction of
carbon monoxide (CO) with lithium amides often gave complex results arising from the incorporation of two CO molecules. Diaminocuprates do not react satisfactorily with CO under atmospheric pressure.
The
lithium-tellurium exchange reaction illustrated here provides a useful alternative to known methods. The reaction can be used to produce various α-hydroxyamides by using aldehydes and ketones as the electrophiles. Trapping the generated carbamoyllithium compounds with esters affords α-ketoamides in good yields (Scheme 1). α-Ketoamides with an aliphatic substituent on the carbonyl carbon cannot be obtained by palladium-catalyzed double carbonylation.
10 Successful trapping of
N,N-diethylcarbamoyllithium with
N,N-diphenylcarbamoyl chloride to give an unsymmetrical oxamide demonstrates a synthetic use of carbamoyllithiums as the umpolung species of carbamoyl halides. A limitation of this procedure may arise from elimination of CO from the carbamoyllithiums. This becomes appreciable when a carbamoyl halide with an aromatic substituent on the
nitrogen is used as a starting material. A variety of N,N-dialkylcarbamoyllithiums, such as N,N-diethyl-, N,N-dimethyl-, and N,N-pentamethylenecarbamoyllithiums can, however, be generated efficiently by the
lithium-tellurium exchange reactions and trapped with various electrophiles to give the corresponding adducts in good yields.
2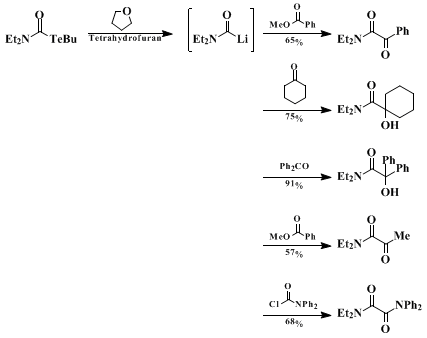
The present procedure provides a convenient method for the generation of carbamoyllithiums from carbamoyl halides and is very useful synthetically for the introduction of carbamoyl groups into organic molecules.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
silica gel
BuTeLi
Et2O
ethanol (64-17-5)
ethyl acetate (141-78-6)
ether,
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
hydrogen (1333-74-0)
carbon monoxide,
formyl hydrogen (630-08-0)
nitric acid (7697-37-2)
nitrogen (7727-37-9)
sodium (13966-32-0)
Naphthalene (91-20-3)
methyl benzoate (93-58-3)
magnesium sulfate (7487-88-9)
butyllithium (109-72-8)
Tetrahydrofuran,
THF (109-99-9)
hexane (110-54-3)
3-phenylpropanal (104-53-0)
tellurium,
tellurium powder
N,N-Diethylcarbamoyllithium
LITHIUM-TELLURIUM
N,N-Diethyl-2-hydroxy-4-phenylbutanamide,
Benzenebutanamide, N,N-diethyl-α-hydroxy- (134970-54-0)
Diethylcarbamoyl chloride (88-10-8)
dibutyl telluride (38788-38-4)
Lithium butanetelluroate
1-phenylheptan-3-ol
N,N-diethylphenylglyoxylamide (34906-86-0)
biscarbamoylmercury
N,N-diisopropylcarbamoyllithium
N,N-diphenylcarbamoyl chloride (83-01-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved