Org. Synth. 1997, 74, 217
DOI: 10.15227/orgsyn.074.0217
MESITYLENESULFONYLHYDRAZINE, AND (1α,2α,6β)-2,6-DIMETHYLCYCLOHEXANECARBONITRILE AND (1α,2β,6α)-2,6-DIMETHYLCYCLOHEXANECARBONITRILE AS A RACEMIC MIXTURE
[Benzenesulfonic acid, 2,4,6-trimethyl-, hydrazide]
Submitted by Jack R. Reid, Richard F. Dufresne, and John J. Chapman
1.
Checked by Michael P. Dwyer and Stephen F. Martin.
1. Procedure
Caution! Mesitylene is an irritant, potassium cyanide is highly toxic, chlorosulfonic acid is corrosive, hydrazine monohydrate is a toxic, cancer-suspect agent, and dichloromethane is an irritant and should be handled in a well-ventilated hood.
A.
Mesitylenesulfonylhydrazine (1). A
500-mL, three-necked, round-bottomed flask is assembled as shown in
Figure 1 (Note 1). The equipment consists of a
250-mL constant pressure addition funnel, a
thermometer, and an
air-cooled Friedrichs condenser attached to a gas
inlet-outlet adapter. A
250-mL vacuum flask is equipped with a
gas delivery tube that is inserted through a
single-holed rubber stopper and positioned 1 cm above the bottom of an
aqueous 20% sodium hydroxide solution. This assembly is used as a gaseous
hydrogen chloride (HCl) trap (Note 2). The flask is charged with
mesitylene (Note 3) (76.0 g, 0.630 mol) and its contents cooled to between −10°C and 0°C in a wet ice–acetone bath while stirring
(Note 4). When the contents of the flask and HCl trap are between −10°C and 0°C, a
6-mL portion of the total quantity of the chlorosulfonic acid (Note 3) (161.0 g, 92.1 mL, 1.39 mol) is added to initiate the reaction. After
HCl gas evolution begins, the remaining
chlorosulfonic acid is added at a rate such that the reaction temperature remains below 60°C
(Note 5). After the
chlorosulfonic acid is added, the reaction mixture is heated to 60°C to disperse and dissolve any precipitated salts
(Note 6) and then allowed to cool to room temperature. The reaction mixture is poured into 250 mL of ice water with stirring, and the crude crystals are recovered by suction filtration. The crystals are washed with generous portions of ice-cold water. The crude product is taken up in
125 mL of dichloromethane and a 50-mL upper aqueous layer is removed. The aqueous layer is extracted with one
50-mL portion of methylene chloride. The combined organic fractions are dried over
sodium sulfate (Note 7) with vigorous stirring, filtered and then concentrated on a
rotary evaporator. Crude
mesitylenesulfonyl chloride (
109.7 g,
80%) is recovered as an oil that solidifies into off-white crystals, mp
54–56°C (Note 8). If used immediately, no additional purification is necessary
(Note 9).
Figure 1
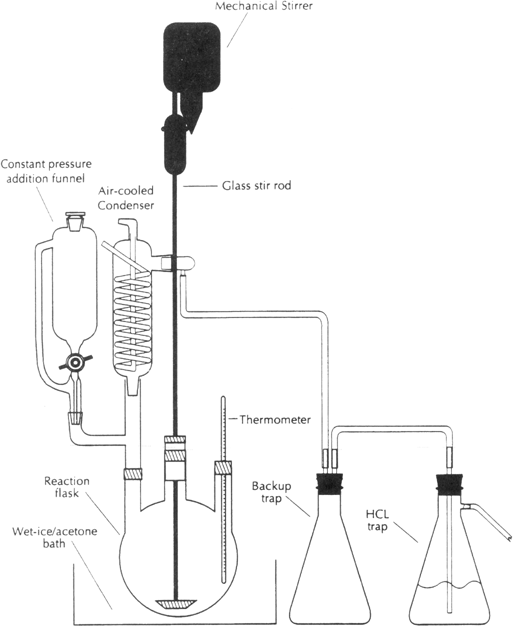
Using the equipment in
Figure 1, but without the HCl gas trap attached,
mesitylenesulfonyl chloride (109.7 g, 0.503 mmol, (Note 7)) is dissolved in
175 mL of dry tetrahydrofuran (THF) and cooled to between −10°C and 0°C in a
wet ice–acetone bath.
Hydrazine monohydrate (63.0 g, 61.0 mL, 1.26 mol) (Note 3) is dissolved in 30 mL of ice-cold water and added so that the temperature remains below 25°C
(Note 10). After the addition is completed, the mixture is stirred for an additional 45 min at room temperature and poured into 250 mL of ice water with stirring. Crude
mesitylenesulfonylhydrazine is recovered by suction filtration and then dissolved in
500 mL of dichloromethane. A 150- to 175-mL portion of water separates. The water layer is removed, and the organic layer is washed with one 50-mL portion of ice-cold water, dried over
sodium sulfate, filtered, and the solvent is removed on a rotary evaporator. The crystals are triturated in
250 mL of cold hexane, and the fluffy white product (
95.4 g,
89%,
(Note 11)) is recovered by suction filtration (
(Note 12) and
(Note 13)).
B.
(1α,2α,6β)-2,6-Dimethylcyclohexanecarbonitrile and (1α,2β,6α)-2,6-Dimethylcyclohexanecarbonitrile (2). The apparatus depicted in
Figure 1 lacking the HCl trap and with a
glass stopper in place of the
thermometer is used for this procedure. The 1-L reaction flask is charged with
mesitylenesulfonylhydrazine (56.0 g, 0.262 mol) (Note 1) and
175 mL of acetonitrile. The mixture is stirred at room temperature until solution is attained
(Note 14).
2,6-Dimethylcyclohexanone (Note 3) (32.2 g, 0.255 mol) is added in one portion and the reactants are stirred an additional 10 min. A 10-drop portion of
concentrated sulfuric acid is added and the mixture is stirred at room temperature for 12 to 18 hr to facilitate complete hydrazone formation
(Note 15). Water is circulated through the reflux condenser that is already attached to the flask, and
potassium cyanide (27.2 g, 0.418 mol) is added
(Note 3). The reaction mixture is gradually heated to reflux over 2 hr and then gently refluxed for 10 to 12 hr
(Note 16). The contents of the flask are cooled to room temperature and water
(125 mL), 20% aqueous sodium hydroxide solution (25 mL),
hexane (175 mL), and
ether (75 mL) are added in succession with vigorous mixing. The mixture is stirred for 15 min, and the lower aqueous layer is separated
(Note 17). The aqueous layer is extracted with a
7:3 hexane-ether mixture (2 × 75 mL). The combined organic layers are washed with two
25-mL portions of aqueous 10% sodium hydroxide solution and dried over
sodium sulfate. The organic layer is filtered and concentrated on a rotary evaporator, and the crude yellow residual oil is distilled at
100–102°C at 39 mm to give
25.5 g (
73%) of colorless
2 (
(Note 18) and
(Note 19)).
2. Notes
1.
The submitters ran the reaction on a scale four times that described. The submitters also note that the reaction may be successfully carried out in a
2-L Erlenmeyer flask equipped with a thermometer to monitor the internal reaction temperature and a large magnetic stirring assembly. The gas delivery tube and rubber stopper, used in
Figure 1, is placed in the mouth of the flask, and the
HCl gas is forced into a base trap or disposed of through an
aspirator.
2.
The HCl trap is charged with
150 mL of aqueous 20% sodium hydroxide solution and cooled in an
ice-water or wet ice-acetone bath.
3.
Mesitylene (1,3,5-trimethylbenzene), chlorosulfonic acid, hydrazine monohydrate, 2,6-dimethylcyclohexanone and potassium cyanide were purchased from Aldrich Chemical Company, Inc., and used without further purification.
4.
A 250-g quantity of wet ice and
250 mL of acetone give a bath temperature between −15°C to −20°C. The bath generally does not need to be recharged.
5.
After half of the
chlorosulfonic acid has been added, the exothermic reaction and gas evolution decrease considerably. If the temperature is allowed to rise above 60°C, the yield decreases and the final product is slightly discolored.
6.
Briefly warming the reaction mixture solubilizes all the
mesitylenesulfonic acid and drives the reaction to completion.
Mesitylenesulfonic acid is less soluble than
mesitylenesulfonyl chloride. Therefore, after everything dissolves, conversion of the acid to the acid
chloride is complete. The product is partially hydrolyzed if it is poured into water while it is still hot.
7.
Methylene chloride partitions water from the crude product in this step and greatly reduces the drying time. However, the mixture is stirred rapidly to bring the water that naturally partitions to the top of the
methylene chloride layer in contact with the drying agent that is on the bottom of the flask.
8.
The spectral properties of the product are as follows:
1H NMR (300 MHz, CDCl
3) δ: 2.35 (s, 3 H), 2.73 (s, 6 H), 7.03 (s, 2 H);
13C NMR (75 MHz, CDCl
3) δ: 21.2, 22.9, 132.3, 139.5, 140.2, 145.3; mp
54–56°C.9.
If
mesitylenesulfonyl chloride is prepared without delay, the damp crystals may be used in the next step without further drying. If the product is to be stored for long periods of time, it is necessary to remove the last traces of water. This is also a convenient place to stop if the reaction will not be completed in a single day.
10.
Temperature control and efficient stirring are more important here than in the preparation of
mesitylenesulfonyl chloride. Better yields are obtained when the temperature is kept below 25°C. Gas evolution accompanied by some decomposition occurs at elevated temperatures.
11.
The spectral and physical properties are as follows:
1H NMR (300 MHz, CDCl
3) δ: 2.31 (s, 3 H), 2.65 (s, 6 H), 3.61 (br s, 2 H), 5.75 (br s, 1 H), 6.99 (s, 2 H);
13C NMR (75 MHz, CDCl
3) δ: 21.0, 22.9, 130.0, 132.1, 140.7, 143.3; mp
114–116°C (lit.
5 mp
115–116°C).
12.
Additional product separates from the filtrate upon standing. Excessive work-up water results in product loss. The crude product can hold considerable volatile material. The weight of the product is usually greater than theoretical prior to thorough air or vacuum drying.
Mesitylenesulfonylhydrazine must be completely dry before it is used in a reaction.
13.
Mesitylenesulfonylhydrazine should be stored in a brown bottle in an area protected from light. Refrigeration of the product, though not required, tends to increase the shelf life.
14.
The checkers noted that gentle warming of the solution with a heat gun greatly facilitated the dissolution of
mesitylenesulfonylhydrazine.
15.
A catalytic amount of
sulfuric acid greatly facilitates formation of the hydrazone which precipitates from solution as it is formed. The checkers found that the reaction mixture must be stirred at room temperature for 14–18 hr to ensure complete formation of the hydrazone. It is advisable to monitor the reaction by TLC. The submitters noted that formation of the hydrazone can be reduced to several hours when the reaction mixture is heated at 40°C.
16.
Unsolvated
potassium cyanide and hydrazone dissolve as the reactants are converted to the thermodynamically favored trans-nitrile. Shortly after all of the solid dissolves, a second solid precipitates from solution. This solid is presumed to be
potassium mesitylenesulfinate and is usually accompanied by some foaming. An oversize flask used with efficient stirring keeps the reaction mixture from foaming into the condenser.
17.
Commercial Clorox, with an activity of 5.25%, is used to destroy excess
potassium cyanide prior to disposing of the aqueous mixture.
18.
A rotary evaporator water bath setting is kept at room temperature during the concentration of the product.
19.
The spectral properties of the product are as follows: IR (CHCl
3) cm
−1: 2933, 2236, 1459, 1384, 1230;
1H NMR (300 MHz, CDCl
3) δ: 0.99 (d, 3 H, J = 7.1), 1.02 (d, 3 H, J = 7.1), 1.37–1.48 (m, 4 H), 1.67–1.72 (m, 1 H), 1.86–1.93 (m, 1 H), 2.00–2.03 (m, 1 H), 2.29–2.33 (m, 1 H);
13C NMR (75 MHz, CDCl
3) δ: 16.5, 19.2, 19.3, 28.9, 30.0, 30.5, 31.2, 40.4, 121.0.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
This is a simplified procedure that uses much less
chlorosulfonic acid than previous methods,
2,3 while obtaining a better yield of
mesitylenesulfonylhydrazine4,5,6 via
mesitylenesulfonyl chloride.
Mesitylenesulfonylhydrazine is used for preparing medium-size cycloalkanones by the Eschenmoser fragmentation reaction,
7 diazo compounds,
8 regiospecific alkylations,
9 and hydrogenations.
5 The hydrolysis of
mesitylenesulfonyl chloride and the decomposition of
mesitylenesulfonylhydrazine is rather slow at low temperatures and short contact times; therefore, the preparations are conveniently worked-up in ice water. However, if damp crystals of
mesitylenesulfonylhydrazine are stored at room temperature for extended periods, an odor of SO
2 becomes evident. Therefore, an alternative procedure for working up very dry
mesitylenesulfonyl chloride and
mesitylenesulfonylhydrazine was developed. This is especially useful if the reaction will not be completed in one session or if the material will be stored for an extended period. The
hydrazine prepared in the first step is used to prepare the racemic product
(1α,2α,6β)- and (1α,2β,6α)-2,6-dimethylcyclohexanecarbonitrile via the corresponding hydrazone in good to excellent yield. This procedure represents a simple, stereoselective, large-scale, one-pot conversion of a moderately hindered ketone to the next higher nitrile analog. The crude product contains small amounts of olefin and starting ketone that can be removed by distillation. Ketones with α,α'-alkyl substituents may be used as diastereomeric mixtures, since they equilibrate to one pair of enantiomers during hydrazone formation, and this stereoselectivity is preserved during the cyanide anion reaction. A minimum of the toxic reagent
potassium cyanide is used and evolution of
hydrogen cyanide is avoided. The use of oxidizing agents such as
bromine and strong bases such as
sodium methoxide in
methanol are avoided, making this method more tolerant of other substituents on the ketone such as olefins.
10,11,12,13,14 The reagent
2,4,6-triisopropylbenzenesulfonylhydrazine is less effective for converting moderately hindered ketones to nitriles, more difficult to prepare, and more expensive to purchase. The nitrile products are useful intermediates in the synthesis of acids by saponification, aldehydes by reduction with
diisobutylaluminum hydride (DIBAL), and ketones by reaction with Grignard reagents.
15,16,17 The scope of this procedure is indicated by the modestly hindered nitriles shown in Table.
TABLE I
KETONES TO NITRILES VIA MESITYLENESULFONYLHYDRAZINE
|
|
Entry
|
Ketone
|
Nitrile
|
Yield,a %
|
Solventc
|
BP (°C/mm)
|
|
|
1
|
4-tert-Butylcyclohexanone
|
4-tert-Butylcyclohexanecarbonitrile
|
73.0–76.7
|
B
|
140–142/30
|
|
2
|
4,4-Dimethylcyclohexanone
|
4,4-Dimethylcyclohexanecarbonitrile
|
73.8
|
B
|
95–97/30
|
|
3
|
4-Isopropylcyclohexanone
|
4-Isopropylcyclohexanecarbonitrile
|
73.0–83.0
|
B
|
130–131/35
|
|
4
|
Pinacolone
|
2,3,3-Trimethylbutanenitrile
|
60.3
|
A
|
54–57/33
|
|
5
|
3,4,4-Trimethylcyclohexanone
|
3,4,4-Trimethylcyclohexanecarbonitrile
|
80.5
|
B
|
127–130/30
|
|
6
|
3,3,5-Trimethylcyclohexanone
|
3,3,5-Trimethylcyclohexanecarbonitrile
|
72.6
|
A
|
110–118/26
|
|
7
|
3-tert-Butylcyclopentanone
|
3-tert-Butylcyclopentanecarbonitrile
|
78.7
|
A
|
117–121/28
|
|
8
|
2,6-Dimethylcyclohexanone
|
2,6-Dimethylcyclohexanecarbonitrile
|
73.5b
|
A
|
97–99/35
|
|
aSatisfactory analytical data, ± 0.3% C, H, were obtained for all of the products.
|
bThe product is 90+% the racemic, and between 1% and 5% the meso nitriles.
|
cSolvent or solvent system used in the reaction: A = acetonitrile; B = 7:1 acetonitrile: 2-methoxyethanol.
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(1α,2α,6β)- and (1α,2β,6α)-2,6-Dimethylcyclohexanecarbonitrile
Mesitylene (1,3,5-trimethylbenzene)
sulfuric acid (7664-93-9)
HCl (7647-01-0)
methanol (67-56-1)
ether (60-29-7)
acetonitrile (75-05-8)
sodium hydroxide (1310-73-2)
chlorosulfonic acid (7790-94-5)
hydrogen cyanide (74-90-8)
bromine (7726-95-6)
sodium sulfate (7757-82-6)
potassium cyanide (151-50-8)
acetone (67-64-1)
sodium methoxide (124-41-4)
Pinacolone (75-97-8)
Mesitylene (108-67-8)
hydrazine monohydrate (7803-57-8)
hydrazine (302-01-2)
mesitylenesulfonic acid
methylene chloride,
dichloromethane (75-09-2)
chloride
Tetrahydrofuran (109-99-9)
hexane (110-54-3)
diisobutylaluminum hydride (1191-15-7)
3,3,5-trimethylcyclohexanone (873-94-9)
4-tert-Butylcyclohexanone (98-53-3)
2,4,6-triisopropylbenzenesulfonylhydrazine (39085-59-1)
Mesitylenesulfonylhydrazine
Benzenesulfonic acid, 2,4,6-trimethyl-, hydrazide (16182-15-3)
Mesitylenesulfonyl chloride (773-64-8)
2,6-Dimethylcyclohexanecarbonitrile
2,6-Dimethylcyclohexanone (2816-57-1)
potassium mesitylenesulfinate
(1α,2α,6β)-2,6-DIMETHYLCYCLOHEXANECARBONITRILE
(1α,2β,6α)-2,6-DIMETHYLCYCLOHEXANECARBONITRILE
4-tert-Butylcyclohexanecarbonitrile
4,4-Dimethylcyclohexanone (4255-62-3)
4,4-Dimethylcyclohexanecarbonitrile
4-Isopropylcyclohexanone (5432-85-9)
4-Isopropylcyclohexanecarbonitrile
2,3,3-Trimethylbutanenitrile
3,4,4-Trimethylcyclohexanone
3,4,4-Trimethylcyclohexanecarbonitrile
3,3,5-Trimethylcyclohexanecarbonitrile
3-tert-Butylcyclopentanone
3-tert-Butylcyclopentanecarbonitrile
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved