Org. Synth. 1992, 70, 195
DOI: 10.15227/orgsyn.070.0195
1,2-ADDITION OF A FUNCTIONALIZED ZINC-COPPER ORGANOMETALLIC [RCu(CN)ZnI] TO AN α,β-UNSATURATED ALDEHYDE: (E)-2-(4-HYDROXY-6-PHENYL-5-HEXENYL)-1H-ISOINDOLE-1,3(2H)-DIONE
[1H-Isoindole-1,3(2H)-dione, 2-(4-hydroxy-6-phenyl-5-hexenyl)-]
Submitted by Ming Chang P. Yeh, Huai Gu Chen, and Paul Knochel
1.
Checked by Thomas Wagler, Brian E. Jones, Thomas C. Zebovitz, and David L. Coffen.
1. Procedure
A. 2-(3-Iodopropyl)-1H-isoindole-1,3(2H)-dione 2. A dry, one-necked, 500-mL, round-bottomed flask equipped with a magnetic stirring bar and a reflux condenser with a gas inlet at the top, is charged with 13.4 g (50 mmol) of 2-(3-bromopropyl)-1H-isoindole-1,3(2H)-dione 1 (Note 1), 17.95 g (120 mmol) of sodium iodide (Note 2), and 100 mL of acetone. The reaction mixture is stirred at reflux under nitrogen for 21 hr (Note 3). The solvent is removed on a rotary evaporator and the resulting solid is dissolved in 300 mL of dichloromethane and 200 mL of water. The two layers are separated in a separatory funnel and the aqueous layer is extracted with two 100-mL portions of dichloromethane. The combined organic extracts are washed successively with 100 mL of an aqueous 10% solution of sodium thiosulfate, three 100-mL portions of water and 150 mL of brine. The organic layer is dried over anhydrous magnesium sulfate. After filtration, the solvent is removed on a rotary evaporator. The crude white solid is dried for several hours at room temperature under reduced pressure to remove traces of solvent [15.0–15.5 g (47.6–49.2 mmol) 95–98% yield]. This material can be used directly in the next step (Note 4).
B.
Formation of the copper-zinc organometallic 3 from 2-(3-iodopropyl)-1H-isoindole-1,3(2H)-dione. A
dry, 100-mL, three-necked, round-bottomed flask is equipped with a magnetic stirring bar, 50-mL pressure-equalizing addition funnel bearing a rubber septum, three-way stopcock, and a thermometer. The air in the flask is replaced by dry
argon and the flask is charged with
4.71 g (72 mmol) of cut zinc (ca. 1.5 × 1.5 mm;
(Note 5)). The flask is again flushed three times with
argon.
1,2-Dibromoethane (Note 6), (0.2 mL, 2.3 mmol) and
3 mL of tetrahydrofuran (THF) (Note 7) are successively injected into the flask which is then heated gently with a
heat gun until ebullition of solvent is observed; the
zinc suspension is stirred a few minutes and heated again. The process is repeated three times;
0.15 mL (1.2 mmol) of chlorotrimethylsilane2 3 4 is then injected into the addition funnel. The cut
zinc foil turns grey. After 15 min the reaction mixture is heated to 30°C with an
oil bath and
18.9 g (60 mmol) of iodide 2 dissolved in
30 mL of THF is added dropwise over 40 min. After addition, the reaction mixture is stirred for 4 hr at 43°C to give a dark brown-yellow solution of the
zinc reagent
(Note 8). A second,
dry, 250-mL, three-necked, round-bottomed flask is equipped with a magnetic stirring bar, three-way stopcock connected to vacuum and an argon source, and two glass stoppers. The flask is charged with
4.59 g (108 mmol) of lithium chloride (Note 9). The flask is heated with an oil bath at 130°C (oil bath temperature) under vacuum (0.1 mm) for 2 hr to dry the
lithium chloride. The reaction flask is then cooled to 25°C and flushed with
argon. The two glass stoppers are replaced by a
low temperature thermometer and a rubber septum and
4.84 g (54 mmol) of copper cyanide (Note 10) is added. The flask is flushed three times with
argon and
40 mL of freshly-distilled THF (Note 7) is added to give, after 15 min, a clear yellow-green solution of the complex CuCN·2LiCl
(Note 11). This solution is cooled to ca. −40°C and the two flasks are connected via a stainless steel cannula. The solution of the
zinc reagent is transferred to the THF solution of
copper cyanide and
lithium chloride (Note 12). The resulting dark green solution is warmed to 0°C within 5 min and is ready to use in the next step after 5 min of stirring at this temperature.
C.
(E)-2-(4-Hydroxy-6-phenyl-5-hexenyl)-1H-isoindole-1,3(2H)-dione 4. The THF solution of the copper-zinc reagent is cooled to −78°C and
19.9 mL (162 mmol) of boron trifluoride etherate (Note 13) is added dropwise. The reaction mixture is warmed to −30°C and stirred for 30 min, then cooled to −60°C.
(E)-Cinnamaldehyde (5.71 g, 43.2 mmol) is added slowly via a syringe. The reaction mixture is allowed to stir at −30°C for 14 hr
(Note 14) and for 30 min at 0°C. After this time, conversion is complete as indicated by GLC analysis and the reaction mixture is poured into an
Erlenmeyer flask containing
500 mL of ethyl acetate,
100 mL of a saturated aqueous solution of ammonium chloride and
5 mL of ammonium hydroxide. The mixture is filtered by suction through
10 g of Celite on a
sintered glass funnel, the contents of the funnel are washed twice with
50 mL of ethyl acetate and the filtrate is separated into two layers. The organic layer is washed successively with
100 mL of aqueous 10% sodium thiosulfate, and twice with
100 mL of a saturated aqueous solution of ammonium chloride. The combined aqueous phases are extracted with
100 mL of ethyl acetate and the combined organic phases are washed with
100 mL of a saturated aqueous sodium chloride solution, then dried over
magnesium sulfate. After filtration, the solvent is removed on a rotary evaporator (ca. 10 mm) to afford
19.85 g of a crude yellowish oil. Flash chromatography separation
5 of the oil using
silica gel (230-400 mesh, 570 g) and
ethyl acetate/hexane (1:2) gives
6.92 g (
50% yield) of the 1,2-addition product
4 as a pale yellow solid, mp
87–88°C, after removal of the solvents
(Note 15).
2. Notes
1.
The
N-(3-bromopropyl)phthalimide 1 was purchased from Aldrich Chemical Company, Inc. or from Lancaster Synthesis Ltd.2.
Sodium iodide (Analytical Reagent) was purchased from Mallinckrodt, Inc.3.
A GLC analysis (Megabore Column (DB5)) shows a conversion of 95%. The remaining bromide
1 is converted to the iodide
2 during the formation of the zinc organometallic (next reaction step).
4.
The iodide
2 can be recrystallized from
hexane/dichloromethane to give white needles; mp
87–88°C.
6 The spectra are as follows: IR (CH
2Cl
2) cm
−1: 3054.6 (m), 2892.5 (w), 1773.8 (m), 1716.1 (s), 1435.8 (s), 1396.3 (m), 1265.6 (s);
1H NMR (360 MHz, CDCl
3) δ: 2.25 (m, 2 H), 3.16 (t, 2 H, J = 7.2), 3.78 (t, 2 H, J = 3.6), 7.34 (dd, 2 H, J = 6.0 and 3.1); 7.86 (dd, 2 H, J = 6.0 and 3.1);
13C NMR (90.5 MHz, CDCl
3) δ: 2.1, 32.6, 38.2, 132.7, 134.8, 168.4.
5.
This procedure uses cut
zinc foil purchased from Alfa Products, Morton/Thiokol Inc. (foil, 0.25 mm thick, 30 cm wide, 99.9% purity). However,
zinc dust can also be used. The reaction time using
zinc dust is shorter and a lower reaction temperature may be possible. After formation of the zinc organometallic, the
zinc dust is allowed to settle and the THF solution of the zinc organometallic is transferred via a syringe to the THF-solution of the complex CuCN·2LiCl. The checkers obtained a
51–56% yield of product, melting at
80–90°C.
6.
1,2-Dibromoethane and the chlorotrimethylsilane are purchased from Aldrich Chemical Company, Inc.7.
All the
tetrahydrofuran used in this procedure was freshly distilled over
sodium/benzophenone before use.
8.
A GLC analysis of hydrolyzed aliquots allows one to check the completion of the reaction. Less than 7% of the starting iodide
2 and more than
93% of N-propylphthalimide can be detected. A yield of 90% of the
zinc reagent is assumed. The
zinc reagent has also been formed at a reaction temperature of 33°C. A reaction time of 16 hr is then required.
9.
Anhydrous lithium chloride is purchased from Aldrich Chemical Company, Inc.10.
Copper cyanide, purchased from Aldrich Chemical Company, Inc., is not a hygroscopic salt and does not need to be dried before use.
11.
A very small amount of undissolved
lithium chloride may still be present and will dissolve after the addition of the zinc reagent.
12.
To effect the transfer, the
argon pressure in the flask containing the copper salt is reduced by inserting a needle through the septum and by shutting off the
argon gas entry. Washing the remaining
zinc foil with
5 mL of dry THF allows one to transfer the zinc reagent almost quantitatively.
13.
Boron trifluoride etherate is purchased from Aldrich Chemical Company, Inc., and is manipulated under
argon.
14.
An
immersion cooler (Cryocool/Neslab) is used to maintain the temperature at −30°C.
15.
A portion of this product is crystallized from
1:1 ethyl acetate:hexane to yield analytically pure product, mp
92–93°C (Anal. Calcd for C
20H
19NO
3: C, 74.75; H, 5.96; N, 4.36. Found: C, 74.52; H, 6.03; N, 4.29 ). The spectra are as follows:
1H NMR (300 MHz, CDCl
3) δ: 1.67–1.81 (m, 4 H), 3.75 (t, 2 H, J = 6.9), 4.34 (m, 1 H), 6.19 (dd, 1 H, J = 15.9 and 6.8), 6.56 (d, 1 H, J = 15.9), 7.22–7.36 (m, 5 H), 7.70 (m, 2 H), 7.82 (m, 2 H);
13C NMR (75.5 MHz, CDCl
3) δ: 24.6, 34.1, 37.7, 72.4, 123.1, 126.4, 127.6, 128.4, 128.5, 130.5, 131.9, 132.0, 133.8, 168.3; IR (CH
2Cl
2) cm
−1: 3489.5 (br), 3058.2 (m), 3024.8 (m), 2942.8 (m), 1771.3 (s), 1709.9 (s), 1467.6 (m), 1438.8 (s), 1398.8 (s), 1337.1 (s), 1266.2 (s), 1069.3 (m), 969.0 (m). MS (EI) m/e 321 (M
+, 45), 304 (5), 263 (2), 216 (24), 189 (20), 174 (87), 160 (89), 156 (25), 148 (33), 133 (100), 115 (30), 105 (41), 91 (44), 77 (45); High resolution MS. Anal. Calcd for C
20H
19NO
3: 321.1365. Found: 321.1369.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Organometallic compounds are among the most versatile intermediates for the formation of carbon-carbon bonds, but their high reactivity allows preparation of only relatively unfunctionalized reagents. In contrast, organozinc halides with a less reactive carbon-metal bond, display a high functional group tolerance, and can include a variety of functional groups such as esters,
7 8 9,10 11 12 13 enoates,
8,14 ketones,
7,15,16 nitriles,
17 18 19 20 halides,
21 22 amino groups,
14,23 24 phosphonates,
25 thioethers,
26 sulfoxides,
26 and sulfones.
26 A transmetallation of these
zinc organometallics to the corresponding
copper compounds, carried out using the THF-soluble
copper salt CuCN·2LiCl, affords highly reactive
copper reagents [RCu(CN)ZnX]. In this procedure, we describe the synthesis of an alkylzinc
iodide with a
phthalimido group at the γ-position, its conversion to the corresponding
copper derivative, and its regiospecific 1,2-addition to
cinnamaldehyde in the presence of
boron trifluoride etherate.
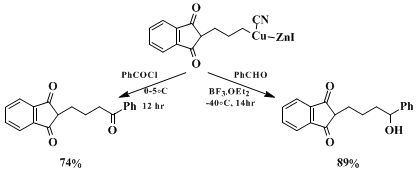
Copper reagent
3 reacts with several other electrophiles in excellent yields (see Scheme). This preparation illustrates the convenient synthesis of highly functionalized organozinc halides in THF
27 and their high synthetic potential.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
silica gel
brine
ethyl acetate (141-78-6)
ammonium chloride (12125-02-9)
sodium chloride (7647-14-5)
sodium thiosulfate (7772-98-7)
nitrogen (7727-37-9)
copper (7440-50-8)
acetone (67-64-1)
Benzophenone (119-61-9)
zinc (7440-66-6)
sodium (13966-32-0)
1,2-dibromoethane (106-93-4)
ammonium hydroxide (1336-21-6)
sodium iodide (7681-82-5)
cinnamaldehyde
dichloromethane (75-09-2)
copper cyanide (544-92-3)
magnesium sulfate (7487-88-9)
iodide (20461-54-5)
Tetrahydrofuran (109-99-9)
hexane (110-54-3)
Lithium chloride (7447-41-8)
argon (7440-37-1)
boron trifluoride etherate (109-63-7)
CHLOROTRIMETHYLSILANE (75-77-4)
phthalimido
(E)-2-(4-Hydroxy-6-phenyl-5-hexenyl)-1H-isoindole-1,3(2H)-dione
1H-Isoindole-1,3(2H)-dione, 2-(4-hydroxy-6-phenyl-5-hexenyl)- (121883-31-6)
2-(3-Iodopropyl)-1H-isoindole-1,3(2H)-dione (5457-29-4)
2-(3-bromopropyl)-1H-isoindole-1,3(2H)-dione,
N-(3-bromopropyl)phthalimide (5460-29-7)
N-propylphthalimide (5323-50-2)
(E)-Cinnamaldehyde (104-55-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved