Org. Synth. 1997, 74, 169
DOI: 10.15227/orgsyn.074.0169
PREPARATION OF POLYQUINANES BY DOUBLE ADDITION OF VINYL ANIONS TO SQUARATE ESTERS: 4,5,6,6a-TETRAHYDRO-3a-HYDROXY-2,3-DIISOPROPOXY-4,6a-DIMETHYL-1(3aH)-PENTALENONE
Submitted by Tina Morwick and Leo A. Paquette
1.
Checked by Lisa Frey and Ichiro Shinkai.
1. Procedure
Caution! tert-Butylithium is extremely pyrophoric and must not be allowed to come into contact with the atmosphere. This reagent should only be handled by individuals trained in its proper and safe use. It is recommended that transfers be carried out by using a 20-mL or smaller glass syringe filled to no more than 2/3 capacity, or by cannula. For a discussion of procedures for handling air-sensitive reagents, see Aldrich Technical Bulletin AL-134. [Note added August 2009]
A 1000-mL, two-necked, round-bottomed flask (Note 1) is equipped with a rubber septum, magnetic stirring bar, and gas inlet connected to a vacuum/argon line via a Firestone valve. The flask is flame-dried under vacuum, then filled with argon. Ten cycles of evacuation and argon fill are carried out. The flask, in which a positive flow of argon is maintained throughout the entire procedure, is charged with 6.66 mL (75 mmol) of 2-bromopropene (Note 2) dissolved in dry tetrahydrofuran (250 mL) (Note 3). The solution is cooled to −78°C by a dry ice/acetone bath, at which point 88 mL of 1.7 M tert-butyllithium in pentane (150 mmol) is introduced dropwise in three roughly equal portions from a 50-mL syringe (Note 4) during 45 min. The colorless reaction mixture is allowed to stir at −78°C for approximately 30 min (Note 5).
A 250 mL, two-necked, round-bottomed flask, equipped with a rubber septum and gas inlet, is flame-dried under vacuum, filled with argon, and charged with a solution of diisopropyl squarate (5.94 g, 30 mmol) (Note 6) in dry tetrahydrofuran (150 mL). The solution is cooled to −78°C and cannulated rapidly into the reaction flask (Note 7). After completion of the addition, the mixture is stirred for 2 hr at 0°C, then for an additional 15 hr at room temperature. Following recooling to 0°C, 180 mL of saturated ammonium chloride solution (Note 8) is added via syringe, the ice bath is removed, and stirring is continued for an additional 7 hr.
The reaction mixture is poured into a 2000-mL separatory funnel containing water (300 mL) and ether (300 mL). After thorough mixing, the aqueous layer is separated and extracted with ether (2 × 200 mL). The combined ethereal solutions are washed with water (300 mL) and brine (300 mL), dried over magnesium sulfate, and evaporated under reduced pressure to leave 8.5 g of a pale yellow oil. This material is subjected to flash chromatography on silica gel using 20% ethyl acetate in hexanes as the mobile phase (Note 9). The minor diquinane (230 mg, (Note 10)) is eluted first. A mixed fraction of the two isomers follows (570 mg, (Note 11)) in advance of the pure title compound (6.36 g, (Note 12), (Note 13)).
2. Notes
1.
All apparatus was washed with base and oven-dried overnight.
2.
2-Bromopropene was purchased from the Aldrich Chemical Company, Inc., and used as received.
3.
Tetrahydrofuran was freshly distilled from
sodium benzophenone ketyl.
4.
tert-Butyllithium was purchased from the Aldrich Chemical Company, Inc., and titrated before use with
diphenylacetic acid according to an established procedure.
2 The syringe and needle were oven-dried overnight prior to use. The plunger is rotated slowly and continuously throughout the addition to avoid loss of mobility brought on by the adventitious formation of
lithium hydroxide.
This alkyllithium is extremely pyrophoric and must be treated cautiously to avoid exposure to the atmosphere.5.
tert-Butyllithium forms a yellow complex with
tetrahydrofuran at low temperatures. When all of this reagent is consumed, the yellow color disappears and a colorless solution of the vinyl anion is obtained. If the color continues the remaining
tert-butyllithium can be consumed by the slow addition of a few drops of
2-bromopropene until a colorless solution results.
6.
Diisopropyl squarate, available from the Aldrich Chemical Company, Inc., can be readily prepared from squaric acid according to the procedure reported by Liebeskind.
37.
An
oven-dried, 18-gauge cannula wrapped with glass wool and aluminum foil was used.
8.
This solution was deoxygenated by bubbling
argon through it for a period of 15 min immediately before use.
9.
The dimensions of the column were 6.5 cm by 36 cm. The relevant R
f values of the isomers are 0.34 and 0.26.
10.
This product, which can be recrystallized from
hexane (colorless crystals, mp
110–111°C), exhibits the following spectral properties: IR (CHCl
3) cm
−1: 3560, 2980, 1650, 1600;
1H NMR (300 MHz, C
6D
6) δ: 0.92–1.12 (m, 1 H), 1.02 (d, 3 H, J = 6.0), 1.03 (d, 3 H, J = 6.0), 1.05 (d, 3 H, J = 6.0), 1.10 (d, 3 H, J = 7.0), 1.15 (d, 3 H, J = 6.0), 1.26 (s, 3 H), 1.25–1.35 (m, 1 H), 1.42–1.50 (m, 1 H), 1.92–2.07 (m, 2 H), 3.08 (s, 1 H), 5.20 (hep, 1 H, J = 6.0), 5.31 (hep, 1 H, J = 6.0);
13C NMR (75 MHz, C
6D
6) δ: 15.3, 19.9, 22.3 (2C), 22.6, 22.8, 30.8, 34.3, 46.6, 52.8, 71.1, 73.4, 83.9, 131.4, 171.9, 199.9.
11.
Assay of this fraction by VPC (SE-30, 70–250°C/min) showed its composition to consist of 8% of the less polar isomer and 92% of the more polar product. The overall yields are consequently 2.5% and 81%, respectively.
12.
The title compound was obtained as a colorless oil that slowly crystallized on standing, mp
52–53°C. Its spectral characteristics are as follows: IR (CHCl
3) cm
−1: 3589, 2979, 1697, 1616;
1H NMR (300 MHz, C
6D
6) δ: 0.84–0.98 (m, 1 H), 1.01 (d, 3 H, J = 7.0), 1.07 (d, 3 H, J = 6.0), 1.09 (d, 3 H, J = 6.0), 1.10 (d, 3 H, J = 6.0), 1.11 (d, 3 H, J = 6.0), 1.28 (s, 3 H), 1.23–1.43 (m, 2 H), 1.86–1.94 (m, 1 H), 2.18–2.24 (m, 2 H), 5.24–5.38 (m, 2 H);
13C NMR (75 MHz, C
6D
6) δ: 15.5, 19.6, 22.5, 22.6 (2 C), 22.9, 31.3, 35.0, 46.9, 56.7, 71.2, 73.6, 83.1, 132.5, 165.4, 202.7.
13.
The checkers isolated an additional side product
A, not observed by the submitters, in 2% yield; its structure is based upon NMR
1H/
13C correlations and NOE data:
1H NMR (300 MHz, CDCl3) δ: 1.2 [(d, 6 H) (OCH(CH3)2)], 2.08 [(m, 3 H) (CH2=C-CH3)], 2.4 [(s, 3 H) (ar-CH3)], 4.3 [(m, 1 H) (OCH(CH3)2)], 5.08 (m, 2 H, =CH2), 5.2 (s, 1 H, 3-OH), 5.6 (s, 1 H, 2-OH), 6.5 (s, 1 H, ar-H).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
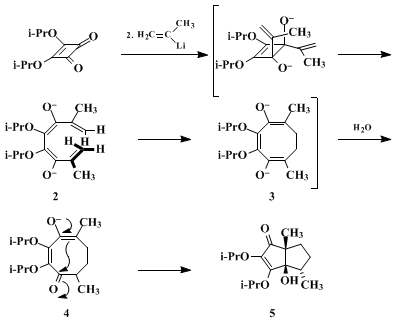
Twofold addition of the same or different vinyl anions to squarate esters leads to polyquinane products.
4 The principal pathway involves trans-1,2-addition of the organometallic reagent to generate a cyclobutene dialkoxide such as
1. Ring strain and electrostatic factors promote the rapid conrotatory opening of
1 in that sense leading to outward splaying of the oxido functional groups.
5 The resultant doubly-charged 1,3,5,7-octatetraenes shown by
2, undergo symmetry-controlled 8π electrocyclization from a coiled conformation, thereby giving rise to cyclooctenyl dienolates
3. In symmetrical examples such as that illustrated, protonation at either available reactive center delivers
4, and sets the stage for intramolecular aldolization via transannular cyclization. In unsymmetrical cases, both aldols are sometimes produced, with steric discrimination occurring.
6 A strong interdependence of the efficiency in this cascade process and vinyl anion substitution has been noted.
6 2-Propenyllithium is particularly conducive to product formation in good yields, presumably because the presence of methyl groups at C-2 and C-7 in
2 favors the adoption of the conformer shown over others less conducive to the conrotatory requirements for the conversion to
3.
The cascade sequence associated with this remarkable series of chemical events is tolerant of structurally varied vinyl anions provided that steric bulk is not excessive. The end result is the potential for establishing many stereogenic centers from a triad of achiral reactants in a single laboratory operation. The very substantial increase in complexity attainable from these tandem stereoregulated chemical events is shown in the Table.
TABLE
POLYQUINANES PRODUCED FROM DIISOPROPYL SQUARATE VIA AN ELECTROCYCLIC CASCADE
|
|
First Anion
|
Second Anion
|
Product (isolated yield)
|
|
|
CH2=CHLi
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2=CHLi
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Two minor processes sometimes operate competitively with that illustrated in the scheme. One of these involves 1,4-addition of the second vinyl anion to give a reactive intermediate that differs structurally from
1, but is capable of setting into motion a closely related sequence of chemical events leading to an isomeric diquinane.
4 This is the route followed to produce the minor product characterized here. The other option consists of cis-1,2-addition, an event that is followed by a dianionic oxy-Cope rearrangement via a boat-like transition state.
4 When sufficient substitution is present to allow the installation of multiple stereogenic centers, the adoption of this pathway is easily distinguished from the electrocyclic alternative since a cis relationship between relevant substituents is in place, instead of the trans arrangement required by the electrocyclization cascade.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
sodium benzophenone ketyl
diquinane
ethyl acetate (141-78-6)
ether (60-29-7)
ammonium chloride (12125-02-9)
Diphenylacetic acid (117-34-0)
Pentane (109-66-0)
magnesium sulfate (7487-88-9)
Tetrahydrofuran (109-99-9)
hexane (110-54-3)
argon (7440-37-1)
2-Propenyllithium
lithium hydroxide (1310-65-2)
2-Bromopropene (557-93-7)
tert-Butyllithium (594-19-4)
diisopropyl squarate
4,5,6,6a-TETRAHYDRO-3a-HYDROXY-2,3-DIISOPROPOXY-4,6a-DIMETHYL-1(3aH)-PENTALENONE
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved