Org. Synth. 1998, 75, 78
DOI: 10.15227/orgsyn.075.0078
SYNTHESIS OF CHIRAL (E)-CROTYLSILANES: [3R- AND 3S-]-(4E)-METHYL 3-(DIMETHYLPHENYLSILYL)-4-HEXENOATE
[
4-Hexenoic acid, 3-(dimethylphenylsilyl)-, methyl ester, [(R)-, and (S)-(E)-]-
]
Submitted by Richard T. Beresis, Jason S. Solomon, Michael G. Yang, Nareshkumar F. Jain, and James S. Panek
1
.
Checked by Pradeep Madan and Steven Wolff.
1. Procedure
A.
(±)-1-(Dimethylphenylsilyl)-1-buten-3-ol
(2a)
. A solution of
10.0 g (0.143 mol) of racemic 3-butyn-2-ol
(Note 1) dissolved in
255 mL of tetrahydrofuran (THF, (Note 2)) in a 1-L, round-bottomed flask equipped with a reflux condenser and nitrogen atmosphere is prepared.
Dimethylphenylsilane (21.4 g, 0.157 mol)
(Note 3) and a small piece of
sodium metal (ca. 5 mg)
(Note 4) are placed in the reaction mixture. The solution is stirred for 15 min and
12 mg (2.05 × 10−5 mol) of bis(η-divinyltetramethyldisiloxane)tri-tert-butylphosphineplatinum(0)
(Note 5) is added. The reaction mixture is then heated under reflux for 12 hr. The orange solution is cooled to ambient temperature, and the solvent is removed under reduced pressure to yield a crude orange residue containing 2a. The oil is subjected to column chromatography on silica gel
(Note 6) (gradient elution
5, 10, 20, 35%
EtOAc/hexanes
) providing 25.4 g (123.23 mmol, 86%) of pure 2a as a yellow oil (Note 7).
B.
(3S)-1-(Dimethylphenylsilyl)-1-buten-3-ol
(3a)
and
(3R)-1-(dimethylphenylsilyl)-1-buten-3-ol acetate
(3b)
. A
400-mL
pentane
solution of
20.0 g (97.08 mmol) of the racemic secondary allylic alcohol
2a
in a
500-mL, round-bottomed flask is prepared. A crude preparation of 10.0 g of lipase Amano AK
(Note 8) is added along with
24.8 mL (269.1 mmol) of freshly distilled vinyl acetate
(Note 9). The heterogeneous mixture is stirred vigorously at 25°C for 4 hr
(Note 10) before being filtered through a
sintered glass funnel to recover the enzyme extract. The extract is washed with
ether (Et2O) (2 × 20 mL). The solvent is removed under reduced pressure and the products are purified by column chromatography on
silica gel: gradient elution
4%
EtOAc/hexanes
affords
11.56 g of acetate (R)-
3b (46.59 mmol,
48%), and gradient elution
20->35%
EtOAc/hexanes
affords
9.3 g of alcohol (S)
-3a (45.12 mmol,
46%), as faint yellow oils
(Note 6),
(Note 11) and
(Note 12).
C.
(3R)-1-(Dimethylphenylsilyl)-1-buten-3-ol
(3c)
.
Acetate (R)-3b
(9.0 g, 36.27 mmol) is dissolved under a
nitrogen atmosphere in a cooled (0°C), 1-L, round-bottomed flask containing
120 mL of anhydrous Et2O
(Note 13). To this stirred mixture is slowly added over 10 min
1.81 g (47.79 mmol, 1.3 eq) of lithium aluminum hydride (LiAlH4). After 15 min, aqueous
5%
hydrochloric acid
is added dropwise until bubbling ceases
(Note 13). The resulting suspension is further diluted with a total volume of 100 mL of the acidic solution. The layers are separated, and the aqueous layer is extracted with
100 mL of Et2O
. The combined organic extracts are washed with an aqueous saturated solution of
sodium chloride
and dried over anhydrous
magnesium sulfate
, filtered, and concentrated under vacuum, to yield
6.27 g (30.42 mmol,
83%) of the (R)-alcohol
3c. No additional purification is performed
(Note 14).
D.
(3S, 4E)-Methyl 3-(dimethylphenylsilyl)-4-hexenoate
(4a)
. A stirred solution of
6.0 g (29.11 mmol) of alcohol
3a
and
60 mL of dry toluene
in a
200-mL flask equipped with a reflux condenser is treated with
15 mL (116.89 mmol) of trimethyl orthoacetate
and
0.15 mL (2.01 mmol, 0.07 equiv) of propionic acid
(Note 15). The reaction mixture is heated under reflux for 48 hr and then allowed to cool to room temperature. Solvents and volatile material are removed under reduced pressure to leave the crude (E)-crotylsilane
4a as a yellow oil. The crude residue is chromatographed on
silica gel (gradient elution
5%
EtOAc/hexanes
). The eluant
(Note 6) affords
6.58 g (25.1 mmol,
86%) of pure
4a as a faint yellow, viscous oil
(Note 6),
(Note 16) and
(Note 17).
2. Notes
1.
3-Butyn-2-ol, 99%, is purchased from Aldrich Chemical Company, Inc.
2.
Tetrahydrofuran is freshly distilled from
sodium
and
benzophenone
.
3.
Dimethylphenylsilane is purchased from United Chemical Technologies, Inc (formerly Hüls Petrarch Inc.).
4.
In the Pt(0)-catalyzed hydrosilation, the addition of a catalytic amount of Na(0) appears to be necessary to achieve high levels of regioselectivity (cf., 1,1- vs 1,2-disubstituted vinylsilane). Without the inclusion of Na(0), the reaction yields a minor regioisomer,
(±)-2-(dimethylphenylsilyl)-1-buten-3-ol,
2b, in a ratio of 10-15:1. The checkers found that the reaction was considerably faster and higher-yielding with
60 mg of the catalyst, bis(η-divinyltetramethyldisiloxane)tri-tert-butylphosphineplatinum(0)
.
5.
The
platinum catalyst is prepared according to the procedure of Chandra and Lo.
2
Chloroplatinic acid, H2PtCl6, (5.0 g, 12.22 mmol) and 0.6 mL of water are added to
61.71 mL (268.84 mmol) of 1,3-divinyltetramethyldisiloxane
. The heterogeneous solution is heated to 55°C in an
oil bath for 4 hr. The now homogeneous solution is cooled to room temperature and
5.0 g of sodium bicarbonate
is added. The solid is filtered, and the resulting yellow solution is treated with
3.33 mL (13.44 mmol) of tri-tert-butylphoshine[(tert-Bu)3P]
. The catalyst precipitates as
4.8 g (
67%) of a white solid that is filtered and washed with
1,3-divinyltetramethyldisiloxane (25 mL), and dried under vacuum. The checkers found that the mixture remained heterogeneous after 4 hr. Heating was continued for 18 hr.
6.
Flash chromatography is performed on E. Merck silica gel 230-400 mesh:
250 g of silica gel
is loaded on a
16- × 2-in size column using a minimum amount of
hexanes as loading solvent.
7.
The hydrosilylation product
2a is sufficiently pure for use as starting material in procedure B. The spectral properties are as follows:
1H NMR (400 MHz, CDCl
3) δ: 0.38 (s, 6 H), 1.30 (d, 3 H, J = 6.5), 4.34 (m, 1 H), 5.99 (dd, 1 H, J = 1.3, 18.7), 6.22 (dd, 1 H, J = 4.9, 18.7), 7.28-7.56 (m, 5 H)
;
13C NMR (67.5 MHz) δ: −2.7(2C), 22.8, 70.4, 125.9, 126.6, 127.6, 128.9, 133.8, 151.3
; IR (neat) cm
−1: 3380, 3080, 2960, 1730, 1620, 1430, 1250, 1110, 860
; CIHRMS M + NH
4+
(calcd for C
12H
22NOSi): 224.1470, (found): 224.1416
.
8.
The amount of enzyme extract is roughly calculated to be 0.5 wt equiv. The crude enzyme preparation may be purchased from Amano Enzyme U.S.A. Co., Ltd., Rt. 2, Box 1475, Troy, VA 22974.
9.
A marked increase in reaction rate is noted when
vinyl acetate is freshly distilled.
10.
Reaction progress is monitored by
1H NMR (CDCl
3); 0.5-mL of sample is withdrawn periodically (at 30-min intervals after 3 hr), filtered through a cotton plug, and the solvent is removed under reduced pressure. The intergration ratio of proton 4.34 (m, 1 H) (S)-
3a, and 5.38 (m, 1 H) (R)-
3b is measured and the reaction is terminated when the integration ratio is 1:1.
11.
The enantiomeric purity of vinylsilane (S)-
3a and (R)-
3c are determined to be >95% ee by
1H-NMR (400 MHz) on the derived mandelate ester, obtained by a DCC-promoted coupling to
(R)-O-acetylmandelic acid
, and absolute stereochemical assignment is accomplished by
1H NMR analysis of the derived (R)-O-acetylmandelate esters. For details of this procedure see the published method of Trost.
3
4
5
12.
The spectral properties are as follows: (S)-
3a:
1H NMR (400 MHz, CDCl
3) δ: 0.38 (s, 6 H), 1.30 (d, 3 H, J = 6.5), 4.34 (m, 1 H), 5.99 (dd, 1 H, J = 1.3, 18.7), 6.22 (dd, 1 H, J = 4.9, 18.7), 7.28-7.56 (m, 5 H)
;
13C NMR (67.5 MHz) δ: −2.7 (2C), 22.8, 70.4, 125.9, 126.6, 127.6, 128.9, 133.8, 151.3
; IR (neat) cm
−1: 3380, 3080, 2960, 1730, 1620, 1430, 1250, 1110, 860
; CIMS (NH
3), m/e (relative intensity): 224 (M+NH
4+
, 100), 206 (M, 44)
; CIHRMS (NH
3), m/e M+NH
4+
(calcd for C
12H
22NOSi): 224.1470, (found): 224.1416
;
[α]
D
23 +4.3° (CHCl
3,
c 0.8); (R)-
3b:
1H NMR (400 MHz, CDCl
3) δ: 0.37 (s, 6 H), 1.32 (d, 3 H, J = 6.5), 2.08 (s, 3 H), 5.38 (m, 1 H), 5.9 (dd, 1 H, J = 1.33, 18.8), 6.1 (dd, 1 H, J = 4.64, 18.8), 7.3-7.5 (m, 5 H)
;
13C NMR δ: −2.7 (2C), 19.8, 21.3, 72.1, 127.7, 127.8, 128.0, 129.0, 133.8, 146.6, 170.2
; IR (neat) cm
−1: 3040, 2950, 1740, 1430, 1570, 1240, 1110, 1040, 830, 740
; CIMS (NH
3), m/e (relative intensity) 209 (33), 179 (24), 171 (68), 135 (100), 117 (97)
; CIHRMS (NH
3), m/e M
+(calcd for C
14H
20O
2Si): 248.1233, (found): 248.1299
;
[α]
D
23 +58.3° (CHCl
3,
c 0.5).
13.
Ether is freshly distilled from
sodium and
benzophenone.
Lithium aluminum hydride is purchased from Aldrich Chemical Company, Inc.
, and used as supplied.
14.
The specific rotation of alcohol (R)-
3c obtained from
LiAlH4
reduction of acetate (R)-
3b is
[α]
D
23 −3.8° (CHCl
3,
c 0.8).
15.
Toluene is freshly distilled from
calcium hydride
.
Trimethyl orthoacetate, 99%, and propionic acid, 99%, are purchased from Aldrich Chemical Company, Inc.
, and used as supplied.
16.
The determination of enantiomeric excess (96% ee) of the Claisen rearrangement products
4a and
4b is accomplished by a Mosher
1H NMR analysis of the (R)-O-acetylmandelate esters that are derived from the primary alcohols. For example,
4a is reduced with LiAlH
4 (1.0 equiv/THF/0°C) followed by esterification of the resulting primary alcohol with
(R)-O-acetylmandelic acid (DCC, 1.5 equiv/cat. DMAP/CH
2Cl
2) to afford the mandelate ester in
91% yield (two steps).
3,4,5
17.
The spectral properties are as follows:
1H NMR (400 MHz, CDCl
3) δ: 0.28 (s, 6 H), 1.64 (d, 3 H, J = 4.7), 2.20 (m, 1 H), 2.27 (dd, 1 H, J = <1.0, 14.5), 2.33 (dd, 1 H, J = 6.3, 14.5), 3.57 (s, 3 H), 5.28 (m, 2 H), 7.35-7.49 (m, 5 H)
;
13C NMR (67.5 MHz) δ: −5.5, −4.5, 18.1, 28.7, 34.3, 51.4, 123.8, 127.7, 129.1, 129.8, 133.9, 136.8, 173.9
; CIMS (NH
3), m/e (relative intensity) 280 (M+NH
4+
, 48), 263 (M
+, 39), 184 (100), 151 (40)
; CIHRMS (NH
3), m/e M+NH
4+
(calcd for C
15H
26NO
2Si): 280.1733, (found): 280.1734
;
[α]
D
23 −11.4° (CHCl
3,
c 0.5) and
[α]
D
23 −15.4° (CH
2Cl
2,
c 1.4) for [(R)-
4b derived from Claisen rearrangement of (R)-
3c. For (S)-
4a
[α]
D
23 +11.9° (CHCl
3,
c 0.5) and
[α]
D
23 +15.9° (CH
2Cl
2,
c 1.6).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Functionalized chiral (E)-crotylsilanes
4a and
4b and related silane reagents have recently been used as carbon nucleophiles in highly diastereo- and enantioselective crotylation reactions.
6 Of particular interest are enantioselective transformations, including an asymmetric [3 + 2] cyclopentane annulation through a conjugate addition and subsequent cyclization. The condensation between the chiral silane reagent and in situ generated N-acylimines affords the homoallylic N-acylamines
7 and functionalized
homoallylic ether formation via a three-component, in situ generation and trapping the derived oxonium ion.
8
9 A subsequent silyl group-directed alkylation of the derived lithium enolate produces a silane reagent bearing functionalization α to the methyl ester which has been employed in the in situ asymmetric crotylsilation producing homoallylic ethers.
10 Equations 1 - 3 illustrate representative examples of the Lewis acid-promoted enantioselective transformations of the silane reagent.
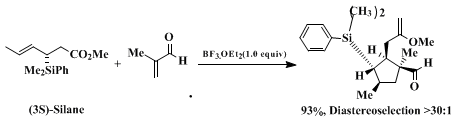
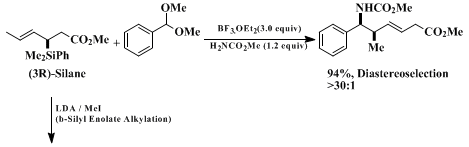
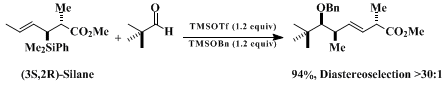
The three-step procedure described for the preparation of the illustrated crotylsilanes is initiated with the hydrosilation of
rac-3-butyn-2-ol. This procedure is significantly improved with respect to the positional selectivity of the hydrosilation resulting in exclusive formation of the racemic
(E)-vinylsilane, and as a result the present procedure is much more amenable to scale-up than those previously described in the literature.
11
12 The enzymatic resolution of the racemic secondary allylic alcohol (
vinylsilane) has also been reported using commercially available lipase extracts. The use of a Johnson ortho ester Claisen rearrangement affords the (E)-crotylsilanes
4 in nearly enantiomerically pure form.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(3R)-(4E)-Methyl 3-(dimethylphenylsilyl)-4-hexenoate:
4-Hexenoic acid,3-(dimethylphenylsilyl)-, methyl ester, [R-(E)]- (12);
(136174-52-2)
(3S)-(4E)-Methyl 3-(dimethylphenylsilyl)-4-hexenoate:
4-Hexenoic acid,3-(dimethylphenylsilyl)-, methyl ester, [S-(E)]- (12);
(136314-66-4)
(±)-1-(Dimethylphenylsilyl)-1-buten-3-ol:
3-Buten-2-ol, 1-(dimethylphenylsilyl)-, (E)-(±)- (12);
(137120-08-2)
3-Butyn-2-ol:
3-Butyn-2-ol, (±)- (10);
(65337-13-5)
Dimethylphenylsilane:
Silane, dimethylphenyl- (8,9);
(766-77-8)
Sodium (8,9);
(7440-23-5)
Bis(η-divinyltetramethyldisiloxane)tri-tert-butylphosphineplatinum(0):
Platinum, [1,3-bis(η2-ethenyl)-1,1,3,3-tetramethyldisiloxane][tris(1,1-dimethylethyl)phosphine]- (11);
(104602-18-8)
(3S)-1-(Dimethylphenylsilyl)-1-buten-3-ol:
3-Buten-2-ol, 4-(dimethylphenylsilyl)-, [S-(E)]- (12);
(133398-24-0)
(3R)-1-(Dimethylphenylsilyl)-1-buten-3-ol acetate:
3-Buten-2-ol,4-(dimethylphenylsilyl)-, acetate, [R-(E)]- (12);
(129921-47-7)
Vinyl acetate:
Acetic acid vinyl ester (8);
Acetic acid ethenyl ester (9);
(108-05-4)
(3R)-1-(Dimethylphenylsilyl)-1-buten-3-ol:
3-Buten-2-ol, 1-(dimethylphenylsilyl)-, [R-(E)]- (12);
(133398-25-1)
Trimethyl orthoacetate:
Orthoacetic acid, trimethyl ester (8);
Ethane, 1,1,1-trimethoxy- (9);
(1445-45-0)
Propionic acid (8);
Propanoic acid (9);
(79-09-4)
1,3-Divinyltetramethyldisiloxane:
Disiloxane, 1,1,3,3-tetramethyl-1,3-divinyl- (8);
Disiloxane, 1,3-diethenyl-1,1,3,3-tetramethyl- (9);
(2627-95-4)
Chloroplatinic acid hexahydrate:
Platinate (2-), hexahydro-, dihydrogen (8);
Platinate (2-), hexachloro-, dihydrogen, (OC-6-11)- (9);
(16941-12-1)
Tri-tert-butylphosphine:
Phosphine, tri-tert-butyl- (8);
Phosphine,tris(1,1-dimethylethyl)- (9);
(13716-12-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved