Org. Synth. 1999, 76, 151
DOI: 10.15227/orgsyn.076.0151
GENERATION AND USE OF LITHIUM PENTAFLUOROPROPEN-2-OLATE: 4-HYDROXY-1,1,1,3,3-PENTAFLUORO-2-HEXANONE HYDRATE
[
2,2,4-Hexanetriol, 1,1,1,3,3-pentafluoro- from 1-Propen-2-ol, 1,1,3,3,3-pentafluoro-, lithium salt
]
Submitted by Cheng-Ping Qian, Yu-Zhong Liu, Katsuhiko Tomooka, and Takeshi Nakai
1
.
Checked by Christine Kim, Peter Belica, and Steven Wolff.
1. Procedure
An oven-dried (Note 1), 300-mL, three-necked, round-bottomed flask containing a magnetic stirring bar, and fitted with a rubber septum inlet, a thermometer and a balloon full of nitrogen is charged with
100 mL of dry tetrahydrofuran
(Note 2) and
6.3 mL (60 mmol) of hexafluoroisopropyl alcohol
(Note 3). The flask and its contents are cooled to −70°C with a dry ice/acetone bath. After the apparatus has cooled,
76.9 mL of a 1.6 M hexane
solution of
butyllithium (123 mmol) is added dropwise using a 100-mL syringe over a period of 15 min. The reaction temperature is kept below −40°C (Note 4). After the addition is complete, the reaction mixture is further stirred for 10 min at −70°C and warmed to 0°C with an ice bath for 1 hr to afford
lithium pentafluoropropen-2-olate
, 1, (Note 5). To the pale yellow solution is added dropwise
4.8 mL (66 mmol) of propionaldehyde
(Note 6) via a syringe over 30 min, and the reaction mixture is stirred for 1 hr at 0°C. The flask is opened to the atmosphere, and about
65 mL of 1.0 M hydrochloric acid
is added for neutralization. The quenched reaction solution is stirred for 10 min at room temperature and then transferred to a 500-mL separatory funnel. The aqueous layer is separated. The organic layer is diluted with
100 mL of ethyl acetate
and washed with
150 mL of saturated brine
. The combined aqueous material is extracted with two
75-mL portions of ethyl acetate
. The organic extracts are combined, dried over anhydrous
magnesium sulfate
, filtered and evaporated. The yellow oily residue is further dried under reduced pressure at room temperature for several hours until crude crystals (about 12-14 g) are formed (Note 7). To the crude crystals is added
20 mL of hexane
, and the resulting material is left at 5°C overnight. After the supernatant liquid is decanted, the wet crystals are washed further with two
20-mL portions of hexane
followed by decantation and dried under reduced pressure to give a first crop of pure crystalline product. The hexane washings are concentrated and treated as described for the oily residue, giving a second crop of pure crystalline product (Note 8). The total yield is 8.64 g (64%) of
4-hydroxy-1,1,1,3,3-pentafluoro-2-hexanone hydrate
(mp 74-78°C) (Note 9).
2. Notes
1.
All the glassware was washed with
acetone and oven-dried for a least 4 hr at 80°C, assembled hot, and allowed to cool under a
nitrogen atmosphere.
2.
Tetrahydrofuran was distilled under a
nitrogen atmosphere from
sodium/benzophenone prior to use.
3.
Hexafluoroisopropyl alcohol was obtained from Central Glass Company, Ltd.
, and used without additional purification. It is advisable to keep this reagent under a
nitrogen atmosphere because it is highly hygroscopic.
4.
During the addition of
butyllithium, a temperature higher than −40°C must be avoided to prevent the formation of a by-product arising from further reaction of the
lithium perfluoroenolate with
butyllithium.
5.
This perfluoro enolate displays the following spectroscopic data:
19F NMR (56.45 MHz, THF, CFCl
3) δ: −68.5 (dd, 3 F, J = 28.6, 9.4, CF
3), −109.8 (br d, 1 F, J = 84.7, CF
2), −117.8 (br d, 1 F, J = 84.7, CF
2)
.
6.
Propionaldehyde was distilled prior to use.
7.
Crystals are difficult to form unless the solvent is removed as completely as possible.
8.
The ratio of the first and second crops, and the total amount of crystallized product depend mainly on whether the
ethyl acetate is removed completely. If necessary, the second
hexane washings can be treated again to give a third crop of product.
9.
The spectral properties of the product are as follows:
1H NMR (300 MHz, DMSO-d
6) δ: 0.94 (t, 3 H, J = 7.5, CH
3), 1.45 (ddq, 1 H, J = 14.0, 9.9, 7.2, CH
2), 1.76 (dddq, 1 H, J = 14.0, 2.7, 2.3, 7.6, CH
2), 3.85-4.05 (m, 1 H, HOCH), 5.96 (d, 1 H, J = 6.5, HOCH), 7.54 [s, 1 H, C(OH)
2], 7.92 [s, 1 H, C(OH)
2]
;
1H NMR (300 MHz, CDCl
3) δ: 1.08 (t, 3 H, J = 7.4, CH
3), 1.60-1.80 (m, 1 H, CH
2), 1.80-2.05 (m, 1 H, CH
2), 2.2-2.9 (br s, 1 H, OH), 3.8-4.4 (br s, 1 H, OH), 4.27 (dddd, 1 H, J = 21.7, 9.9, 3.1, 1.4, HOCH), 5.4-6.3 (br s, 1 H, OH)
;
13C NMR (75 MHz, DMSO-d
6) δ: 9.9 (CH
3), 22.4 (CH
2), 71.0 (dd, J = 27.7, 22.7, HOCH), 92.6 [m, C(OH)
2], 118.5 (t, J = 256.5, CF
2), 122.6 (q, J = 289.3, CF
3)
;
19F NMR (56.45 MHz, AcOEt, CFCl
3) δ: −80.0 (dd, 3 F, J = 13.5, 11.7, CF
3), −120.0 (ddq, 1 F, JF
A-F
B = 275.3, JF
A-H = 2.0, JF
A-CF
3 = 13.5, CF
AF
B), −132.0 (ddq, 1 F, JF
B-F
A = 275.3, JF
B-H = 21.9, JF
B-CF
3 = 11.7, CF
A
F
B)
; IR (neat film/NaCl plate) cm
−1: 3400, 2980, 1440, 1200, 1100, 1070, 980, 920, 850, 770, 750
. Anal. Cacld for C
6H
9F
5O
3: C, 32.15; H, 4.05; F, 42.38. Found: C, 32.09; H, 3.96; F. 42.04.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
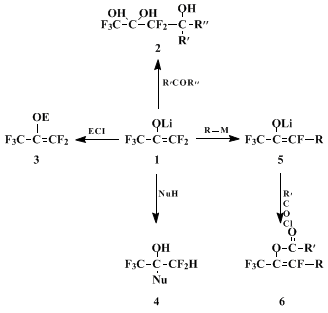
The present procedure describes an extremely convenient preparation of
lithium perfluoropropenolate (
1) and illustrates its use in organofluorine synthesis. The following scheme shows the wide spectrum of reactivity observed with lithium F-enolate
1.
2,3,4 Several features of the F-enolate reactivity have been demonstrated: (a) The F-enolate exhibits the usual enolate reactivities, i.e., O- and C-nucleophilicity, to afford various classes of polyfluorinated compounds of types
2~4 that are otherwise difficult to obtain. (b) The F-enolate is capable of undergoing aldol reactions with various carbonyl compounds to afford adducts as hydrates
2. (c) In reactions with reagents bearing an active
hydrogen, the initial protonation occurs at the β-carbon, not at the oxygen, to yield products of type
4. (d) Most significantly, the F-enolate exhibits a rather unusual electrophilic reactivity toward organometallic reagents to generate a geometric mixture of the β-alkyl F-enolates
5 via an addition-elimination process. This means that the F-enolate behaves as a kind of perfluoroolefin that is well-known to undergo a similar type of substitution reaction. Some examples of these reactions are shown in Tables I and II.
Finally, it should be noted that the present procedure is applicable to preparations of various lithium F-enolates such as
7 and
8 that exhibit different reactivity from that of
1.
3,4
TABLE I
REACTION OF LITHIUM PENTAFLUOROPROPEN-2-OLATE WITH ELECTROPHILES
|
|
Electrophile
|
Product (% Yield)
|
Electrophile
|
Product (% Yield)
|
|
|
EtCHO
|
|
Me3SiCI
|
|
|
PhCHO
|
|
(MeO)2SO2
|
|
|
PhCOMe
|
|
H2O
|
|
|
CF3CHO
|
|
PhCH2OH
|
|
|
(C2F5)2CO
|
|
PhCONH2
|
|
|
PhCOCI
|
|
CHF(CO2Et)2
|
|
|
TABLE II
REACTION OF LITHIUM PENTAFLUOROPROPEN-2-OLATE WITH NUCLEOPHILES
|
|
Nucleophile
|
Producta
|
Yield (%)
|
|
|
BuLi
|
|
71
|
|
PhLi
|
|
64
|
|
PhMgBr
|
|
48
|
|
Red-AI
|
|
55
|
|
aMetal enolates 5 thus formed were isolated after acylation with an acyl chloride.
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Lithium pentafluoropropen-2-olate:
1-Propen-2-ol, 1,1,3,3,3-pentafluoro-, lithium salt (12); (116019-90-0)
4-Hydroxy-1,1,1,3,3-pentafluoro-2-hexanone hydrate:
2,2,4-Hexanetriol, 1,1,1,3,3-pentafluoro- (12); (119333-90-3)
Hexafluoroisopropanol alcohol:
2-Propanol, 1,1,1,3,3,3-hexafluoro- (8,9); (920-66-1)
Butyllithium:
Lithium, butyl- (8,9); (109-72-8)
Propionaldehyde (8);
Propanal (9); (123-38-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved