Org. Synth. 1999, 76, 228
DOI: 10.15227/orgsyn.076.0228
USE OFCERIUM(III) CHLORIDE IN THE REACTIONS OF CARBONYL COMPOUNDS WITH ORGANOLITHIUMS OR GRIGNARD REAGENTS FOR THE SUPPRESSION OF ABNORMAL REACTIONS:1-BUTYL-1,2,3,4-TETRAHYDRO-1-NAPHTHOL
Submitted by Nobuhiro Takeda and Tsuneo Imamoto
1
.
Checked by Scott Ballentine and David J. Hart.
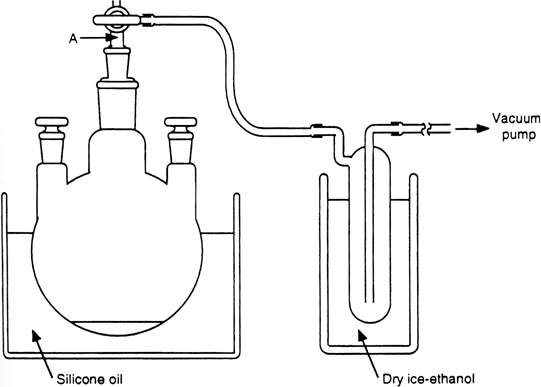
1. Procedure
1-Butyl-1,2,3,4-tetrahydro-1-naphthol
. A
500-mL, three-necked, round-bottomed flask is equipped with two glass stoppers and a three-way stopcock
(Note 1) and
(Note 2). The flask is connected to a
trap
(Note 3) that is cooled at −78°C in a dry
ice-ethanol bath and attached to a
vacuum pump (
Figure 1). The flask is charged with powdered
cerium(III) chloride heptahydrate (CeCl3·7H2O, 44.7 g, 0.12 mol)
(Note 4) and evacuated to 0.1-0.2 mm. After gradual warming to 90°C over 30 min with an
oil bath, the flask is heated at 90-100°C for 2 hr with intermittent shaking
(Note 5). The system is filled with dry
argon and cooled to room temperature. The solid is transferred to a
mortar and quickly pulverized with a
pestle. The resulting white powder and a
magnetic stirring bar
(Note 6) are placed in the original flask. Gradual warming to 90°C at 0.1-0.2 mm over 30 min, followed by further evacuating at 90-100°C for 1.5 hr with intermittent shaking, gives
cerium(III) chloride monohydrate (CeCl3·H2O)
(Note 7). The
cerium(III) chloride monohydrate is gradually warmed to 140°C over 30 min under reduced pressure (0.1-0.2 mm) without stirring
(Note 8). Heating at 140-150°C/0.1-0.2 mm for 2 hr with gentle stirring
(Note 9) affords a fine, white powder of anhydrous
cerium(III) chloride
(Note 10). While the flask is still hot, the area that is not immersed in the oil bath is heated by the use of a heat gun in order to remove traces of water. After introduction of
argon into the flask, the resulting anhydrous
cerium(III) chloride is cooled to room temperature. One of the glass stoppers is replaced by a
rubber septum in a stream of dry
argon.
Figure 1. Apparatus for the preparation of anhydrous cerium(III) chloride.
Tetrahydrofuran (200 mL)
(Note 11) is added to the powder of anhydrous cerium(III) chloride in one portion at 0°C in an ice-water bath with vigorous stirring (Note 12). After the mixture is stirred under argon atmosphere at room temperature overnight (Note 13), the resulting milky suspension is cooled to −78°C with a dry ice-ethanol bath. A 1.54 M hexane solution of
butyllithium (78 mL, 0.12 mmol) is added dropwise over 15 min to the suspension, so that the temperature of the reaction mixture remains at −78°C. The resulting yellow suspension is stirred at the same temperature for 30 min, and
α-tetralone (13.5 mL, 14.8 g, 0.101 mol)
(Note 14) is added to the mixture at −78°C over 15-30 min. After the mixture is stirred at the same temperature for 30 min, the dry ice-ethanol bath is removed, and the pale yellow suspension is warmed to room temperature. An aqueous
5% solution of acetic acid (200 mL) is added to the suspension with vigorous stirring. The reaction mixture is transferred to a 1-L separatory funnel, and
ethyl acetate (100 mL) is added. The organic layer is separated, and the water layer is extracted with
ethyl acetate (2 × 50 mL). The combined organic layers are washed sequentially with
brine (50 mL), a saturated aqueous solution of
sodium bicarbonate (50 mL), and
brine (50 mL) and dried over anhydrous
magnesium sulfate
. After evaporation of the solvent by a rotary evaporator under reduced pressure, distillation of the residual oil gives 19.1-19.9 g of
1-butyl-1,2,3,4-tetrahydro-1-naphthol
(92-97%) (Note 15) and (Note 16) as a colorless oil, 103-104°C/0.5 mm.
2. Notes
1.
The joints of the apparatus are lubricated with silicone grease to avoid permeation of moisture from outside. All temperatures refer to external temperatures.
2.
Neither cotton nor glass wool should be attached to part "A" of the three-way stopcock (
Figure 1), because adhesion of
cerium(III) chloride to them, caused by bumping of the powder, prevents reducing the pressure.
3.
The trap is attached in a reverse manner to prevent clogging of the trap by ice.
4.
Cerium(III) chloride heptahydrate was purchased from Japan Yttrium Co., Ltd. or Aldrich Chemical Company, Inc.
, and ground in a mortar with a pestle just before use.
5.
The temperature of the oil bath must be kept below 100°C to minimize the hydrolysis of
cerium(III) chloride heptahydrate to CeOCl.
6.
A
powerful magnetic stirrer and a
large rugby ball-shaped stirring bar are used.
7.
When the resulting
cerium(III) chloride (730-779 mg) is dissolved in
10 mL of dry methanol
, Karl Fischer analysis of the solution shows that the
cerium(III) chloride contains 6.5-7.6% of water which is equal to 95-113% of the water expected from the formula CeCl
3·H
2O.
8.
If the
cerium(III) chloride powder is stirred at this stage, it sprays around the flask. This causes not only the loss of a large amount of
cerium(III) chloride, but also clogs of the three-way stopcock with the powder.
9.
A small amount of
cerium(III) chloride is lost by bumping; however, it does not affect the yield of the product.
10.
Karl Fischer titration of a dry
methanol solution (15 mL) of the resulting
cerium(III) chloride (731-817 mg) shows that it contains 0.71-0.94% of water and can be represented by a formula CeCl
3(H
2O)
0.10-0.13. This result indicates that this procedure affords practically anhydrous
cerium(III) chloride, although Evans, et al. reported that gradual heating to 150°C at 0.03 mm over 3 hr, followed by further evacuating at 150°C/0.03 mm for 12 hr, resulted in the production of
cerium(III) chloride monohydrate.
2
Anhydrous cerium(III) chloride can be stored in a sealed vessel. It is dried under reduced pressure (0.1-0.2 mm) at 140-150°C for 1 hr just before use.
11.
Tetrahydrofuran was freshly distilled under
argon from
sodium and
benzophenone.
12.
Addition of
tetrahydrofuran to anhydrous
cerium(III) chloride without either cooling or vigorous stirring results in the formation of a hard cake, which prevents the formation of the milky suspension.
13.
Instead of stirring overnight, the suspension may be prepared by sonication for more than 1 hr.
3
4
14.
α-Tetralone was purchased from Tokyo Kasei Kogyo Co., Ltd. or Aldrich Chemical Company, Inc.
, and distilled under reduced pressure before use.
15.
The preparation of anhydrous
cerium(III) chloride
5
6 is necessary to obtain a satisfactory result. For example, the use of
cerium(III) chloride monohydrate in the same reaction causes a significant lowering of the yield (
34%) of the desired alcohol (the recovery of the starting ketone: 54%). The use of the reverse addition procedure (see Method B in the Table) also resulted in the formation of the alcohol in poor yield (36%; the recovery of the ketone: 58%).
TABLE
CERIUM(III) CHLORIDE-PROMOTED CARBONYL ADDITIONSa
|
|
Entry
|
Ketones
|
Reagents
|
Methodb
|
Products
|
Yield (%)c,d
|
|
|
1
|
α-Tetralone
|
BuLi
|
|
|
26
e (55
e)
|
|
2f
|
α-Tetralone
|
BuLi/CeCI3
|
A
|
same as above
|
92-97
|
|
3
|
α-Tetralone
|
BuLi/CeCI3
|
B
|
same as above
|
80
e (11
e)
|
|
4
|
α-Tetralone
|
BuMgBr
|
|
same as above
|
28
e (23
e)g
|
|
5
|
α-Tetralone
|
BuMgBr/CeCI3
|
A
|
same as above
|
96
|
|
6
|
α-Tetralone
|
BuMgBr/CeCI3
|
B
|
same as above
|
92
e (2
e)
|
|
7h
|
PhCH=CHCOPh
|
MeLi
|
|
PhCH=CHC(OH)(Me)PH
|
54
|
|
|
|
|
|
Ph(Me)CHCH2COPh
|
22
|
|
8h
|
PhCH=CHCOPh
|
MeLi/CeCI3
|
A
|
PhCH=CHC(OH)(Me)Ph
|
97
|
|
|
|
|
|
Ph(Me)CHCH2COPh
|
1
|
|
9i
|
p-IC6H4COMe
|
BuLi
|
|
p-IC6H4C(OH)(Me)Bu
|
trace
|
|
10i
|
p-IC6H4COMe
|
BuLi/CeCI3
|
A
|
p-IC6H4C(OH)(Me)Bu
|
93
|
|
11h
|
PhCH2CO2Me
|
i-PrMgBr
|
|
PhCH2COCH(Ph)CO2Me
|
71 (10)
|
|
|
|
|
|
PhCH2COPr-i
|
19
|
|
|
|
|
|
PhCH2C(OH)(Pr-i)2
|
0
|
|
12h
|
PhCH2CO2Me
|
i-PrMgBr/CeCI3
|
A
|
PhCH2COCH(Ph)CO2Me
|
0
|
|
|
|
|
|
PhCH2COPr-i
|
0
|
|
|
|
|
|
PhCH2C(OH)(Pr-i)2
|
97
|
|
13h
|
Et3CCOMe
|
MeMgBr
|
|
Et3CC(OH)Me2
|
0
|
|
14h
|
Et3CCOMe
|
MeMgBr/CeCI3
|
A
|
Et3CC(OH)Me2
|
95
|
|
aThe reactions with organolithiums and Grignard reagents are carried out at −78°C and 0°C, respectively.bMethod A: the carbonyl compound is added to the mixture of CeCI3 and the organolithium or Grignard reagents; Method B: the organolithium or Grignard reagent is added to the mixture of CeCI3 and the carbonyl compound.cIsolated yield unless otherwise stated.dThe figures in parentheses indicate the yields of the recovered starting material.gDetermined by1H NMR.fThe reaction procedure is described in the text.gThe reduction product, α-tetralol, is formed in 39% yield.hSee Ref. 9.iSee Ref. 8.
|
16.
The spectral and physical data of this material are as follows: IR (neat) cm
−1: 3390
;
1H NMR (400 MHz, CDCl
3) δ: 0.88 (t, 3 H, J = 7.3), 1.10-1.40 (m, 4 H), 1.70-2.05 (s, 7 H), 2.65-2.85 (m, 2 H), 7.00-7.07 (m, 1 H), 7.10-7.22 (m, 2 H), 7.45-7.52 (m, 1 H)
;
13C NMR (75.4 MHz, CDCl
3) δ: 13.9 (q), 19.7 (t), 23.1 (t), 26.3 (t), 29.9 (t), 35.9 (t), 42.1 (t), 72.4 (s), 126.09 (d), 126.12 (d), 126.9 (d), 128.8 (d), 136.7 (s), 142.4 (s)
; Anal. Calcd for C
14H
20O: C, 82.30; H, 9.87. Found: C, 82.13; H, 9.97.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Reactions of carbonyl compounds with organolithiums or Grignard reagents normally give the corresponding alcohols via nucleophilic addition to the carbonyl group.
12
13 Nevertheless, the reactions are often accompanied by so-called abnormal reactions such as enolization, reduction, condensation, conjugate addition, and pinacol coupling. Several attempts to suppress these undesired reactions have been made by changing solvents
14
15
16 or using additives.
17
18
19
20
21 These methods are not always efficient, although they are effective in some cases. On the other hand, the use of
cerium(III) chloride as an additive results in the efficient suppression of these abnormal reactions,
7,8 and many applications of this method to practical organic syntheses have been reported.
9
10
11
A typical example of an effective synthesis of a tertiary alcohol from an easily enolizable ketone by taking advantage of the organolithium/cerium(III) chloride system is illustrated here, along with experimental details for the preparation of anhydrous cerium(III) chloride. The reaction of α-tetralone with butyllithium alone affords 1-butyl-1,2,3,4-tetrahydro-1-naphthol in poor yield (26%), because of enolization, which gives the starting ketone (55%) (Table, Entry 1). The efficient production of the desired alcohol (92-97%) in the reaction with butyllithium/cerium(III) chloride (Table, Entry 2) suggests the lower basicity of this system. The use of butylmagnesium bromide instead of butyllithium in this reaction also results in the efficient transformation of the starting ketone to the corresponding alcohol (Table, Entry 5). The reaction with butylmagnesium bromide/cerium(III) chloride can be performed at 0°C, in contrast to the reaction with butyllithium/cerium(III) chloride at 0°C, which gives a complex mixture. The reverse addition procedure (Table, Method B) affords the desired alcohol in moderate or satisfactory yield (Table, Entries 3 and 6).
This method is also effective in enhancing 1,2-addition to α,β-enones (Table, Entries 7 and 8) as well as suppressing metal-halogen exchange (Table, Entries 9 and 10) and aldol-type condensation (Table, Entries 11 and 12). Furthermore, this is applicable to the reaction of sterically congested ketones (Table, Entries 13 and 14). Various organolithiums and Grignard reagents such as alkyl, alkenyl, alkynyl, and aryllithiums and magnesium halides can be employed for this method.
7,8,9,10,11 The reactions with these organolithiums and Grignard reagents are carried out at −78°C and 0°C, respectively, although vinylic Grignard reagents are treated with
cerium(III) chloride at −78°C because of the rapid decomposition of the Grignard reagents on contact with
cerium(III) chloride at 0°C.
8
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Cerium(III) chloride heptahydrate:
Cerium chloride heptahydrate (8);
Cerium chloride (CeCl3), heptahydrate (9); (18618-55-8)
Cerium(III) chloride monohydrate:
Cerium chloride (CeCl3), monohydrate (10); (64332-99-6)
Cerium(III) chloride, anhydrous:
Cerium chloride (8,9); (7790-86-5)
Butyllithium:
Lithium, butyl- (8,9); (109-72-8)
α-Tetralone:
1(2H)-Naphthalenone, 3,4-dihydro- (8,9); (529-34-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved