Org. Synth. 1999, 76, 294
DOI: 10.15227/orgsyn.076.0294
9,10-DIPHENYLPHENANTHRENE
[
Phenanthrene, 9,10-diphenyl-
]
Submitted by George A. Olah, Douglas A. Klumpp, Donald N. Baek, Gebhart Neyer, and Qi Wang
1
.
Checked by Vladimir Dragan and David J. Hart.
1. Procedure
Caution! Triflic acid is a highly corrosive liquid and should be handled carefully.
9,10-Diphenylphenanthrene
. In a 250-mL, three-necked, round-bottomed flask equipped with a magnetic stirrer, a thermometer, and a septum is suspended
10.0 g (27.3 mmol) of benzopinacol
(Note 1) in
40 mL of dry toluene
(Note 2) under a nitrogen atmosphere. The suspension is cooled to 0°C using an ice-acetone bath, and
40 mL of freshly distilled trifluoromethanesulfonic acid (triflic acid)
(Note 3) and (Note 4) is added in one portion via syringe (Note 5). The cold bath is removed, and the mixture is stirred for 24 hr at room temperature. The resulting heterogeneous mixture is poured over 100 g of ice, the organic layer is separated, and the aqueous layer is extracted with two
100-mL portions of toluene
. The organic layers are combined, and washed with water (50 mL) and with brine (50 mL). The original solution is dried with anhydrous
magnesium sulfate
, filtered, and the filtrate is concentrated under reduced pressure to afford 9.2 g of crude product (mp 227-231°C) as a pale yellow solid. The crude product is recrystallized from
450 mL of toluene-ethanol
(1:1) to give 6.7 g (74%) of pure
9,10-diphenylphenanthrene
as a white solid (Note 6). Concentration of the mother liquor and recrystallization of the residue from
100 mL of toluene-ethanol
(1:1) gives another 1.4 g (16%) of product (mp 237-238°C).
2. Notes
1.
Benzopinacol was purchased from Aldrich Chemical Company, Inc.
, and used without further purification.
2.
Toluene was purchased from Fisher Scientific Company
and distilled from
sodium prior to use.
Benzene may be substituted for
toluene with the following differences: (a) The initial solution is cooled to only 10°C, otherwise the
benzene layer freezes. (b) The mixture is extracted with three
100-mL portions of benzene
after pouring over ice. (c) Recrystallization from
430 mL of benzene-ethanol
(1:1) gives a first crop of pure product in
80-90% yield. The submitters indicate that
dichloromethane can be used as the solvent for the reaction and extractions.
3.
Triflic acid was obtained from 3M (99% purity). The
triflic acid must be distilled prior to its use, and the distillation should be performed under a dry, inert atmosphere.
Triflic acid may be recycled using a published procedure.
2
4.
Experiments indicated that the acid strength of the reaction medium must be H
o = −12 or stronger. Pure
triflic acid has been estimated to be about H
o = −14.
3 Contamination of the
triflic acid by water results in a weakened acid system. When the acid strength drops below H
o = −12, significant quantities of
2,2,2-triphenylacetophenone
are produced from the pinacol rearrangement.
5.
The temperature of the reaction mixture increases by approximately 15°C during the addition of
triflic acid.
6.
This material exhibited the following properties: mp
237-238°C (lit.
4 238°C);
1H NMR (300 MHz, CDCl
3) δ: 7.13-7.26 (m, 10 H), 7.5 (t with fine coupling, 2 H, J = 6.9), 7.6 (d with fine coupling, 2 H, J = 8.4), 7.7 (t with fine coupling, 2 H, J = 6.6), 8.82 (d, 2 H, J = 8.1)
;
13C NMR (75 MHz, CDCl
3) δ: 122.9, 126.8, 126.9, 127.1, 128.0, 128.3, 130.4, 131.5, 132.3, 137.6, 139.9
; HRMS calcd for C
26H
18
330.1408, found 330.1408
.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
When aromatic pinacols are reacted with an acid, products often arise from dehydration and rearrangement.
5
6
7 This general conversion is known as the pinacol rearrangement. The pinacol rearrangement may be promoted by both Brönsted and Lewis acids.
8
9 In the procedure described here, superacidic
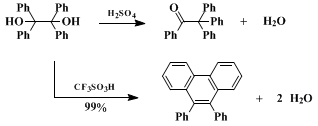
triflic acid is reacted with an aryl pinacol and a dehydrative cyclization occurs to give the substituted
phenanthrene product. Related to this conversion, the chemistry of
benzopinacol in
sulfuric acid and
triflic acid is contrasted in Scheme 1. We have proposed that the superacidic
triflic acid causes the formation of diprotonated intermediates which promote the dehydrative cyclization.
4
As described in an earlier report,
4 it has been demonstrated that the dehydrative cyclization of aryl pinacols in
triflic acid is a general route to substituted phenanthrenes. Some of these conversions are described in the Table. Entries 5-7 show an interesting aspect of the conversion. When two of the aryl rings are substituted by strongly deactivating groups such as chlorine or bromine, the product is formed regioselectively. It may be that the chlorine or bromine deactivate the rings toward cyclization, and that the cyclization is occurring more rapidly through the phenyl or alkylphenyl rings. When two aryl rings are substituted by weakly deactivating groups such as fluorine, poor regiochemistry is observed, and cyclization occurs through both the phenyl and fluorophenyl rings, giving rise to complex product mixtures.
TABLE.
SUBSTITUTED PHENANTHRENES FROM THE DEHYDRATIVE CYCLICATION OF ARYL PINACOLS IN TRIFIC ACID
|
|
Entry
|
Pinacol
|
Product
|
Yieldb
|
|
|
1
|
|
|
99%
|
|
2a
|
|
|
90%
|
|
3a
|
|
|
95%
|
|
4a
|
|
|
100%
|
|
5a
|
|
|
98%
|
|
6a
|
|
|
87%
|
|
7a
|
|
|
94%
|
|
aAryl pinacol prepared by the photolysis of the appropriately substituted benzophenone in isopropyl alcohol.
|
bYields reported for the crude product.
|
Aryl pinacols are readily obtained from the photolysis of substituted benzophenones in
isopropyl alcohol
. Photochemical coupling of benzophenones in
isopropyl alcohol has even been reported to occur in direct sunlight.
10 It is known however, that some aryl ketones do not give the
pinacol from photolysis in
isopropyl alcohol.
11 Nevertheless, aryl pinacols have also been prepared from electrochemical reduction,
12 hydroxylation of olefins,
13 photoreduction of ketones by amines,
14
15
16 metal reduction,
17
18
19
20 and by other routes.
21 The phenanthrene ring system has often been prepared by the photochemical cyclization and oxidation of stilbenes,
22 and
9,10-diphenylphenanthrene has been previously synthesized by the reaction of
1,2-dichlorotetraphenylethane
with excess
aluminum chloride
.
23
24 The present method provides a direct route to substituted phenanthrenes from readily available aryl ketones via the aryl pinacol.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
9,10-Diphenylphenanthrene:
Phenanthrene, 9,10-diphenyl- (8,9); (602-15-3)
Benzopinacol:
1,2-Ethanediol, 1,1,2,2-tetraphenyl- (8,9); (464-72-2)
Trifluoromethanesulfonic acid: HIGHLY CORROSIVE:
Methanesulfonic acid, trifluoro- (8,9); (1493-13-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved